Metal Alloys (GCSE Chemistry)
Metal Alloys
Metal Alloys
Everyday Uses
- Alloys are everyday metals. A lot of metals used day-to-day are alloys. This means that they are a mixture of two or more metals.
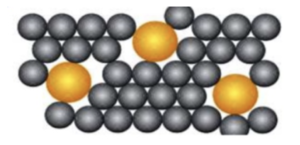
- Alloys have different properties. Since alloys are mixtures of metals, this means that they will have different properties depending on the metals that they contain. The different sized atoms disrupt the metal layers making them harder to slide. They will also be harder than pure metals.
Common Alloys
- Bronze is made from copper and tin. Bronze is an alloy that can be used for decorating ornaments and statues, and it is made from copper and tin.
- Brass is made from copper and zinc. Brass is soft, so it can be used for taps. This allows water to flow with less friction. It is an alloy of copper and tin.
Gold Alloys
- Gold can form alloys. Gold can be mixed with silver, copper and zinc to form an alloy. By forming an alloy, pure gold (which is relatively soft), becomes harder.
- Gold alloys can be used for jewellery. The strengthened gold alloy is commonly found in jewellery. It is a very valuable metal.
- Gold is measured in carats. We can measure the amount of gold in an alloy by using carats. Pure (100%) gold is 24 carats, whilst 18 carats means that the alloy contains 75% gold.
Steel Alloys
- Steel is an alloy of iron. As we’ve seen previously, steel is an alloy of iron. It contains carbon and other metals as well as iron.
- Carbon proportions can vary. The proportion of carbon in a steel alloy can vary. When there is a lot of carbon, high carbon steel, contains 0.5 to 2% carbon. The steel is very strong but brittle. This type of steel can be used to build bridges.
- Low carbon steel (0.05 to 0.25% carbon) is soft. Steel alloys containing a low proportion of carbon is very soft and therefore easily shaped. This type of steel can be used to make cars.
- Chromium and nickel resist corrosion. If the steel alloy contains chromium and nickel, this means that it is hard and resists corrosion. This type of steel is called stainless steel, usually used to make cutlery.
Aluminium Alloys
- Aluminium alloys are low density. Alloys that involve aluminium are low density. This means that they are lightweight.
- Aluminium is used in drinks cans. Due to its lightweight but strong nature, aluminium is used in drinks cans.
- Duralumin is an alloy containing aluminium. Duralumin is an alloy made of aluminium, copper and magnesium. It has a low density but a higher strength in comparison to aluminium alone, so is used in aircraft construction.
A metal alloy is a mixture of two or more metals, or a metal and another element, that is created by melting and mixing the components together.
Metal alloys are used because they often have improved properties compared to pure metals, such as increased strength, durability, and corrosion resistance.
Steel is an alloy made primarily from iron and carbon. It is made by melting iron and adding carbon and other elements such as chromium or nickel to create the desired properties.
Brass is an alloy made from copper and zinc. It is made by melting the two metals together in the appropriate proportions.
Bronze is an alloy made from copper and tin. It is made by melting the two metals together in the appropriate proportions.
Ferrous alloys contain iron as the main metal, while non-ferrous alloys do not contain iron.
An amalgam is an alloy made from a metal and mercury. It is made by mixing the two components together.
Examples of metal alloys include steel, brass, bronze, stainless steel, and titanium.
An alloy is a mixture of two or more metals or a metal and another element, while a pure metal is made up of only one type of metal.
Metal alloys often have improved properties compared to pure metals, such as increased strength, durability, and corrosion resistance, due to the addition of other elements.
Metal alloys are used in everyday life for a wide variety of applications, such as in construction, transportation, electronics, and medical devices.





Still got a question? Leave a comment
Leave a comment