Collision Theory & Activation Energy (GCSE Chemistry)
Collision Theory & Activation Energy
Collision Theory
- Collisions transfer energy. When particles collide, they transfer energy. In order for a collision to be successful, the particles must collide with the necessary activation energy.
- Collision theory is linked to rate of reaction. Collision theory helps to explain how different factors affect the rate of a reaction. If a higher number of collisions occur between particles in a given time, ie the collisions are more frequent between particles, the rate of reaction will be higher.
Increasing the Rate of Reaction
The rate of reaction is affected by the five different factors mentioned previously. Here, we will look at how these factors can increase the rate of a reaction.
All of the factors will increase the rate of reaction by creating a higher number of collisions in a given time between the particles. The particles that collide successfully will cause a reaction.
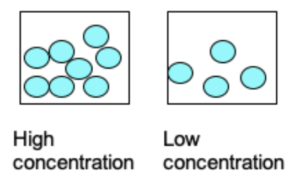
Increasing the Concentration of Reactants
When the concentration of the reactants is increased, there is an increased number of particles contained in the same volume of liquid.
Since there are more particles in the same volume, there is a higher frequency of reactant collisions or more collisions in a given time. In turn, this causes an increase in the rate of reaction.
In coal mines, the concentration of methane gases is very high. If a certain concentration is reached, methane can react with air to form an explosive mixture.
Increasing the Pressure of Reacting Gases
When the pressure of a reacting gas is increased, the same number of particles are contained in the smaller volume of gas.
Since there is a smaller amount of space in which the particles can move about, there is a higher frequency of reactant collisions. In turn, this causes an increase in the rate of reaction.
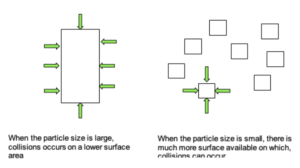
Increasing the Surface Area of Solid Reactants
When the surface area of a solid reactant is increased, this is due to the reactant being broken down into many small pieces. This process will increase the surface area to volume ratio of the reactant.
Since there is a larger area of solid with which the particles can react, there are more frequent reactant collisions. In turn, this will cause an increase in the rate of reaction.
A larger surface area can make a solid more combustible. Fine powders from industrial processes have a larger surface area, so are more combustible and therefore more at risk of forming an explosive mixture with air. For example flour in flour mills and wood dust in saw mills.
Increasing the Temperature
Increasing the temperature of a reaction will increase the rate of reaction in two ways:
- Particles move more quickly. When the temperature increases, this makes particles move around more quickly. This results in a greater frequency of particle collisions.
- Particles have more energy. The particles will have more energy due to their faster movement. More of the particles will therefore have the necessary activation energy, leading to more successful particle collisions.
Collision theory in GCSE Chemistry is a scientific explanation of how chemical reactions occur. It explains that chemical reactions only occur when the particles collide with enough energy and in the right orientation.
Activation energy in GCSE Chemistry refers to the minimum amount of energy required for a chemical reaction to occur. The activation energy is the energy barrier that the particles must overcome in order to react with each other.
Activation energy and collision theory in GCSE Chemistry are closely related. According to collision theory, a chemical reaction only occurs when the particles collide with enough energy to overcome the activation energy barrier.
Temperature affects activation energy in GCSE Chemistry because increasing the temperature of a reaction increases the energy of the particles, making it more likely that they will collide with enough energy to overcome the activation energy barrier and react.
Concentration affects activation energy in GCSE Chemistry because increasing the concentration of the reactants increases the number of particles present, making it more likely that they will collide with each other and react.
The concepts of collision theory and activation energy in GCSE Chemistry have a wide range of real-world applications, including:
Understanding the behavior of catalysts: Catalysts can be used to lower the activation energy of a reaction, making it more likely to occur at a lower temperature.
Improving industrial processes: By understanding how collision theory and activation energy impact chemical reactions, industrial processes can be optimized for greater efficiency and reduced energy consumption.
Studying biological processes: Collision theory and activation energy can also be used to explain the behavior of biological systems, such as enzymes and metabolic pathways.





Still got a question? Leave a comment
Leave a comment