Graphene & Fullerenes (GCSE Chemistry)
What is Graphene?
- Graphene is a single layer of graphite. Graphene is made up of a single layer of hexagonal rings, with each carbon atom bonded to three other carbon atoms meaning each carbon atom has one delocalised electron (like graphite). Graphene is a single carbon atom thick forming a two-dimensional sheet.
- Graphene is very strong and has a low density. Graphene is extremely strong due to the many, strong covalent bonds between the carbon atoms. These covalent bonds are difficult to overcome and require a lot of energy. Graphene is also very light. These properties allow graphene to be added to other substances to increase their strength without increasing their weight forming a composite.
- Graphene can conduct electricity. Graphene properties include electrical conduction because it contains delocalised electrons which are free to move throughout the single sheet of carbon atoms and carry charge. This property means graphene can be used in electronics.

What is graphene used for?
Graphene has many uses in various different sectors, including transport, biomedicine, electronics, clothing, construction and energy. A few examples include targeted drug delivery, electrical components, coating on clothing, membranes for construction materials and energy consumption when charging objects such as phones and cars.
What are Fullerenes?
Fullerenes are molecules of carbon atoms that form large hollow shapes. Fullerenes are made of carbon atoms that join together to form hollow hexagonal (6C) rings, sometimes these structures can also have pentagonal (5C) and heptagonal (7C) carbon rings. They form large hollow shapes such as cages or cylinders.
Buckminsterfullerene (C60)
- The first fullerene discovered was Buckminsterfullerene (C60). Buckminsterfullerene was the first fullerene to be discovered. It consists of 60 carbon atoms covalently bonded together forming a spherical shape.
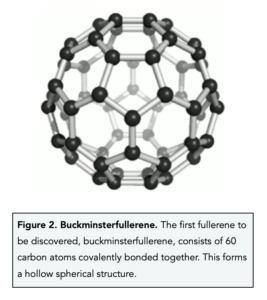
- Buckminsterfullerene (C60) has a low melting point. Buckminsterfullerene has a low melting point because between molecules of buckminsterfullerene there are weak intermolecular forces that require little energy to overcome.
- Buckminsterfullerene (C60) is slippery. Buckminsterfullerene is slippery because between molecules of buckminsterfullerene there are weak intermolecular forces which allow molecules of this fullerene to slide past each other.
Carbon Nanotubes
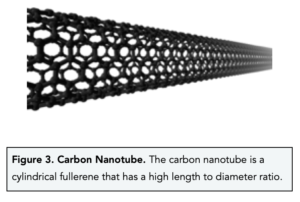
- Carbon nanotubes are cylindrical fullerenes. Fullerenes can form thin cylinders known as carbon nanotubes. These nanotubes are much longer than they are wide, therefore they have a high length to diameter ratio.
- Carbon nanotubes have high tensile strength. Nanotubes are able to withstand high tension when being stretched and do not break. This is because they have a very high length to diameter ratio.This property is useful when making composite materials which can be used for tension racquets.
- Carbon nanotubes are good conductors of electricity. Like graphene, carbon nanotubes can conduct electricity because it contains delocalised electrons which are free to move throughout the single sheet of carbon atoms and carry charge. This property means these nanotubes can be used in electronics.
What can fullerenes be used for?
Fullerenes are being used within the field of medical science. This includes being used as light-activated antimicrobial agents. It is also used in medical imagery as contrast agents in MRIs and x-rays.
Predicting Structure
You should learn the properties of graphene and fullerenes, and the following main types of bonding as you could be asked to predict the structure of a random substance based on its properties.
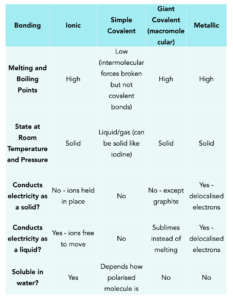
Practice Question: An unknown substance conducts electricity only when molten and dissolved in solution. It has a relatively high boiling point of 1245K. What is the type of structure that this substance has?
Answer: It can only conduct electricity when molten and dissolved in solution. This applies to all ionic compounds as when they are molten or dissolved, the ions are free to move around and carry charge/
It also has a high melting point. We know that ionic bonding involves a strong electrostatic attraction between oppositely charged ions. This means a large amount of energy is needed to overcome the forces.
The unknown substance must have an ionic structure.
Graphene and Fullerenes are two types of carbon-based materials that have unique properties and potential uses in various industries. Graphene is a single layer of carbon atoms arranged in a honeycomb lattice, while Fullerenes are balls or tubes made of carbon atoms.
Graphite is made up of many layers of graphene stacked on top of each other, while graphene is just one single layer of carbon atoms. This single layer of graphene gives it unique properties that make it different from graphite.
Graphene has many remarkable properties, such as being extremely strong, lightweight, flexible, and an excellent conductor of heat and electricity. It is also transparent and has high chemical stability.
The potential uses of Graphene are numerous and diverse, including in electronics, energy storage, water purification, and even biomedical applications. Graphene is also being explored for use in the creation of new materials and products that are stronger, lighter, and more efficient.
Fullerenes are known for their unique structure and unique properties, such as being good conductors of electricity and excellent lubricants. They also have potential uses in fields such as electronics, energy storage, and medicine.
Graphene and Fullerenes have significant importance in the field of chemistry because of their unique properties and potential uses. They have opened up new avenues for scientific research and development, and have the potential to revolutionize many industries and improve our lives in many ways.
Graphene can be made by exfoliating graphite or by growing it on a substrate using chemical vapor deposition. Fullerenes are made through the process of arc discharge or laser ablation. Both methods require specialized equipment and knowledge, and are typically carried out in a laboratory setting.





Still got a question? Leave a comment
Leave a comment