Chromatography and Rf Values (GCSE Chemistry)
Chromatography
- Chromatography can separate mixtures. By using chromatography, we can separate mixtures. This will help us to identify the substances within the mixture.
- Chromatography has two phases. There are two phases in chromatography, called the mobile phase and the stationary phase. These phases allow the mixture to separate.
- The two phases reach an equilibrium. As compounds move through the two phases of chromatography, an equilibrium is formed.
- Molecules can move in the mobile phase. The mobile phase can be a liquid or a gas, allowing molecules to move.
- Molecules cannot move in the stationary phase. The stationary phase can be a solid or thick liquid, which doesn’t allow the molecules to move. In this phase, molecules can separate out of the mixture.
- Distribution between phases determines separation. Depending on the distribution of the compound between the mobile and stationary phases, molecules will separate.
Rf Values
- Substances start at the origin. The starting point of the compound is called the origin. This is where we measure the distance moved by the substance.
- Substances move different distances. Each substance will move a different distance. We can measure the distance travelled by substance by going from the centre of the spot to the origin.
- Rf values are ratios. An Rf value is the ratio of the distance moved by the compound as compared to the distance moved by the solvent. If the solvent only moves a short distance, then the Rf value will be small.

Differences Between Rf Values
- Rf values can differ. Rf values are not the same for every substance and every solvent. The Rf value will change depending on the solvent used.
- Rf values identify compounds. Due to the differing Rf values, substances can be identified by chromatography. This allows us to see the substances contained within one compound by comparing them to reference values.
- Substances create a series of spots. When chromatography is carried out on a compound, the substances separate out to create a series of spots. Each spot will represent one specific substance.
- Pure compounds produce a single spot. When we carry out chromatography on a pure compound, one single spot will be formed. This indicates that there are no other substances present, reinforcing that the compound is pure.
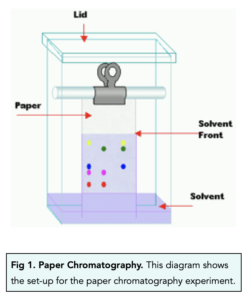
Paper Chromatography
- Paper chromatography separates mixtures. Paper chromatography is a form of chromatography. By using chromatography, we can separate mixtures and identify compounds.
- Paper chromatography identifies pure substances. As previously mentioned, we can identify pure substances from chromatograms, as they produce one single spot on the chromatogram.
- Paper chromatography has two phases. Paper chromatography has a mobile phase and a stationary phase. The mobile phase is the solvent, whilst the stationary phase is the chromatography paper.
- Paper chromatography gives Rf values. Like any other form of chromatography, we can calculate Rf values from paper chromatography using the equation we saw previously.
- Rf values are affected by the solvent. In paper chromatography, the Rf values are affected by the solvent used. The substances will move at different rates depending on how soluble they are in the solvent, and how attracted they are to the paper.
- Paper chromatography can identify colourless substances. By adding locating agents to chromatograms with colourless substances, coloured products or ones which glow under ultraviolet light can be formed. An example is iodine vapour. It turns brown when reacted with fats and oils.
Using Paper Chromatography
- Paper chromatography separates mixtures. As we’ve seen, paper chromatography is a form of chromatography. By using chromatography, we can separate mixtures and identify compounds.
- Paper chromatography separates coloured substances. Paper chromatography can be used to separate the compounds within coloured substances such as pen ink.
Method
- Gather equipment. For this experiment, we will need a solvent, some substances (in this case, different coloured inks), filter paper, a container, a pencil, a capillary tube, and a ruler.
- Draw the origin. Using the ruler and a pencil, draw a straight line about 2cm from the bottom of the filter paper. This is the start line, where the ink droplets to be separated will be placed. A graphite pencil is used as graphite is insoluble and will not spread in water.
- Add ink spots. Using the inks and a capillary tube, place a single spot of each ink on the start line. Using a capillary tube will make sure that the spots of ink are all a similar size and on the start line. Make sure the spots are far enough apart so they will not spread into each other.
- Place the paper into the solvent. Put a small amount of solvent into the container, then place the tip of the filter paper with the ink spots into the solvent. Make sure to place the paper upright into the container and ensure that the solvent is below the ink spots.
- Place a lid onto the container. Make sure to place a lid onto the container so that the solvent does not evaporate.
Worked example: Look at the chromatogram in the diagram above.
Draw three conclusions about the three substances analysed by the chromatogram.
Answer: Any three from:
Substance A has four different substances, as there are four spots.
Substance B has three different substances, as there are three spots.
Substance C has three different substances, as there are three spots.
Substance A and B both have two of the same substances (red and pink spots).
Substances A and C both have two of the same substances (blue and yellow spots).
Substances B and C both have one of the same substances (green spot).
Worked example: Look at the chromatogram given here. Calculate the Rf value of the red spot.
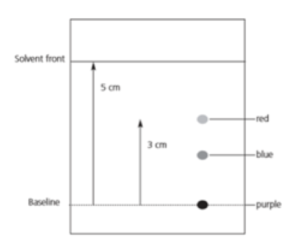
Answer: Rf value = distance travelled by the spot/ distance travelled by the solvent = ⅗ = 0.6
Chromatography is a technique used to separate and identify the components of a mixture. It works by using the different physical and chemical properties of the components to separate them.
The Rf value, also known as the retention factor, is a measure of the position of a component in a chromatographic separation. It is calculated by dividing the distance travelled by the component by the distance travelled by the solvent.
The Rf value is important in Chromatography because it allows us to identify the components of a mixture. By comparing the Rf value of a component in a mixture to the Rf values of known compounds, we can determine the identity of the component.
The Rf value of a component in Chromatography can be affected by several factors, including the type of stationary phase, the polarity of the solvent, the temperature, and the concentration of the components in the mixture.
To calculate the Rf value in Chromatography, you divide the distance travelled by the component by the distance travelled by the solvent. For example, if the component travels 5cm and the solvent travels 10cm, the Rf value is 0.5.
Chromatography works by using the different physical and chemical properties of the components in a mixture to separate them. The mixture is applied to a stationary phase, such as a piece of paper or a column, and a solvent is added. The components in the mixture move at different speeds through the stationary phase based on their properties, allowing them to be separated.
There are several different types of Chromatography, including Paper Chromatography, Thin Layer Chromatography (TLC), Column Chromatography, and Gas Chromatography (GC). Each type uses a different stationary phase and a different method for separating the components of a mixture.
Chromatography is used in a variety of real-life applications, including the analysis of food and drink, the identification of drugs and chemicals, and the separation of proteins and enzymes in biochemistry. It is also used in environmental testing to detect and measure pollutants in air, water, and soil.





Still got a question? Leave a comment
Leave a comment