Potable Water (GCSE Chemistry)
Uses of Water
Inadequate Water Supply
Not having a clean, adequate water supply can lead to adverse consequences:
- Food shortages are more likely to occur. A lack of water means crops cannot be irrigated, meaning they will not grow. This leads to a lack of food and could eventually cause a famine.
- Inadequate water supplies can lead to poor sanitation. Without good sanitation, disease is more likely to spread, especially if drinking water becomes infected.
Drinking Water
Potable Water
- Water is essential for life. Human life requires water, as long as it is safe to drink. This water must be of good quality.
- Drinking water is ‘potable water’. Potable water is another name for drinking water. This water has low levels of dissolved salts and microbes.
- Potable water contains dissolved substances. Potable water is not ‘pure’ water, because it contains dissolved substances.
Potable Water Production
Production Methods
- Production methods of drinking water vary. In different parts of the UK, there are different methods for producing drinking water. This depends on various factors and conditions.
- Water supply is an important factor. In the production of drinking water, the supply of water is an important factor. Another important consideration is the local conditions in an area.
Using Rainwater
- Rainwater is used to produce potable water. We use rainwater in the UK to produce our drinking water. The rainwater contains very low levels of dissolved substances, meaning it is fresh water. Rain water collects in rivers, lakes and as ground water.
- We can collect groundwater or surface water. We can collect rainwater from rocks under the ground. This is called groundwater. We can also collect rainwater from lakes and rivers, known as surface water.
- Surface water can dry up. Since surface water is found in lakes and rivers, these can dry up in warmer weather. This means that in hotter climates and areas, drinking water sources are predominantly groundwater.
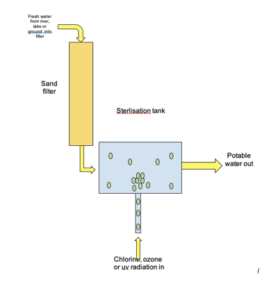
- To remove insoluble solids, rainwater is passed through filter beds. Sand filters are used to remove small insoluble solids from fresh water.
The Sterilisation Process
- Rainwater must be sterilised. Before using rainwater is used as potable water, it must be sterilised. This is because freshwater contains a small amount of bacteria.
- Filtration is the first step in the process. The first part of the process to create potable water is filtration. This is done by passing fresh water through filter beds to remove any large particles like stones, or small particles like sand.
- Sterilising agents are then used. After the filtration process, sterilising agents are then used to treat the rainwater. These sterilising agents include chlorine gas, ozone and ultraviolet light. These kill microbes to reduce levels so they are sufficiently low.
Using Seawater
- Surface water and groundwater may not be available. In some extremely hot countries, there may not be any surface water or groundwater available to make potable water.
- Sea water can be used instead. Instead of using rainwater, sea water can be used to create potable water. This water is extremely salty. We need to remove this salt from the water.
- Reverse osmosis can remove the salt. By using a process called reverse osmosis, we can remove the salt from sea water. A membrane is used to do this. High pressure is applied to the sea water, to push water molecules through the membrane; the salt is left behind.
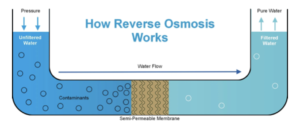
- Using sea water can be expensive. The process of using sea water to create potable water is very expensive. This is because reverse osmosis uses a lot of energy to produce high pressure.
- Desalination can be produced by distillation of sea water. In distillation, sea water is heated to over 100°C. The water vapour produced does not contain salt. It is cool and condenses to make pure water through desalination. This is an expensive process, as lots of energy is needed to boil large volumes of water.
Analysis and Purification of Water Samples
For exams, you’ll need to know how water samples can be analysed and purified. We’ll look at a method of detecting pH, dissolved solids and distillation here.
Method
1.Test the pH of water. First, we must test the pH of the water. We can do this using a pH meter to check whether the water is acidic or alkaline.
2. Measure the concentration of dissolved solids.
- Take a clean, dry watch glass and weigh it.
- Dry by warming the watch glass in an oven to constant mass.
- Place a known volume e.g. 5 cm3 of water sample on the watch glass.
- Heat the water glass over a water bath until all the water evaporates.
- Remove the watch glass and solids and dry.
- Dry by warming in an oven to constant mass.
- Weigh the watch glass and solids.
- Calculate the mass of solids by subtracting the mass of the dry watch glass.
- Divide the mass by the volume of water to find the concentration.
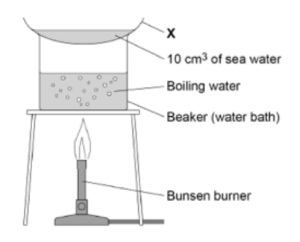
3. Analysing sea water using distillation. The apparatus below can be used to analyse samples of seawater by distillation. Salt is left in the flask and pure water is produced as the distillate.
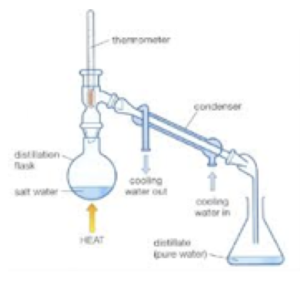
Worked example: Evaluate the ease with which it is to obtain potable water from ground water and salt water. (6 marks)
Model answer:
Potable water has sufficiently low levels of dissolved salts and microbes to be safe to drink.
Ground water: This is a suitable fresh water source of potable water. Water has already been filtered by rocks and needs less filtration. It will need to be sterilised with chlorine, or ozone, or ultraviolet light to kill microbes and pathogens.
Salt must be removed from seawater either by distillation or reverse osmosis. Both processes need large amounts of energy. Distillation needs large amounts of energy to heat up water to 100°C, followed by cooling and condensing the sea water. The water produced has no salt. Alternatively, reverse osmosis can be used to remove salt from sea water. This is achieved by using high pressure and forcing the sea water through special membranes. Again, the water produced is pure.
Overall ground water is most suitable as it requires the least number of processes and the least amount of energy to make the water potable. Salt water would be the most expensive and create the largest carbon footprint.
Waste Water
Waste Water Treatment
- Urban lifestyles produce waste water. Through urban lifestyles and industrial processes, humans create a lot of waste water. Some examples include sewage, washing-up water and waste water from fields.
- Waste water requires treatment. The waste water in sewers requires treatment before it can go back into the environment. We must remove any harmful substances from the water.
- Organic matter, microbes and chemicals must be removed. More specifically, we must remove high levels of organic matter, harmful microbes and chemicals from waste water. These could have entered the water system through industrial processes, sewage and agricultural processes.
Sewage Treatment
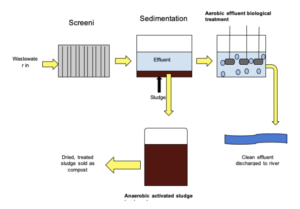
- Waste water requires treatment. As we’ve seen, waste water in sewers requires treatment before it can go back into the environment. We can do this using a four step process, outlined here.
- Sewage screening. Before the waste water can be properly treated, any large particles must be removed from the water. This is done through a process called screening, which removes large particles and grit.
- Sedimentation. We can then let the waste water stand in a tank. This will let any heavy particles sink down towards the bottom of the tank. This process is called sedimentation, where sludge sinks to the bottom and effluent floats to the top.
- Bacterial treatment. We can then use bacteria to treat the effluent from the waste water. This bacteria will start a process of aerobic digestion, which breaks down any microbes in the water.
- Anaerobic digestion. Now that the effluent has been treated, the sludge should be treated. This is done via the process of anaerobic digestion, which releases methane gas. Methane gas can be used as an energy source.
Potable water is water that is safe to drink and use for cooking and other household purposes. It is free from harmful contaminants and meets health and safety standards.
Potable water is treated in several ways to make it safe to drink, including:
Coagulation and flocculation: This involves adding chemicals to cause impurities in the water to clump together and become easier to remove.
Sedimentation: The clumps of impurities are allowed to settle to the bottom of a tank, where they can be removed.
Filtration: The water is then filtered through sand or other materials to remove any remaining impurities.
Disinfection: Finally, the water is disinfected to kill any remaining harmful bacteria or viruses, usually through the addition of chlorine.
Some of the contaminants commonly found in water that need to be treated before it can be considered potable include:
Bacteria and viruses
Heavy metals, such as lead and mercury
Chemicals, such as pesticides and herbicides
Minerals, such as iron and manganese
Organic matter, such as leaves and animal waste
The easiest way to determine if water is potable is to have it tested by a water treatment professional. They can perform tests to check for the presence of harmful contaminants and ensure that the water meets health and safety standards.
To ensure the water you drink is potable, you can:
Use a home water treatment system, such as a water purification pitcher or faucet-mounted filter.
Boil the water for at least one minute to kill any harmful bacteria or viruses.
Have your water tested by a professional to ensure it meets health and safety standards.
Access to potable water is essential for good health and well-being. Drinking contaminated water can lead to the spread of diseases and illnesses, making access to safe and clean drinking water a fundamental human right.






Still got a question? Leave a comment
Leave a comment