Catalysts (GCSE Chemistry)
Catalysts
Catalysts
- Catalysts increase the rate of reaction. By using a catalyst, the rate of reaction can be increased. Catalysts are not part of the reaction equation, since they are not used up in the reaction.
- Catalysts decrease the activation energy. Catalysts will increase the rate of reaction by providing a different pathway for the reaction to occur. This pathway has a lower activation energy, therefore making it easier for the reaction to occur.
- Different reactions require different catalysts. Catalysts are reaction specific, which means that we must use different catalysts for different reactions. Enzymes are examples of biological catalysts, which act as catalysts for living things.
Reaction Profiles for Catalysed Reactions
Similar to drawing the reaction profile of a reaction, we can also draw the reaction profile for a catalysed reaction as well.
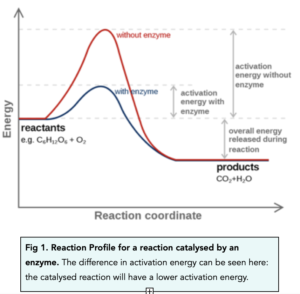
In a catalysed reaction, the activation energy is at a lower level. This means that less energy is needed for a reaction to take place. More particles are therefore likely to have enough energy for successful collisions to occur.
Effect of different solids on the catalytic decomposition of hydrogen peroxide solution
You need to be able to describe an experiment to determine the effect of different catalytic solids on the decomposition of hydrogen peroxide solution.
Method
- Gather your equipment. You will need a conical flask, capillary tube, measuring cylinder, container, bung, a solution of hydrogen peroxide and different solid catalysts.
- Add hydrogen peroxide into a flask. Add a known volume of hydrogen peroxide into a conical flask.
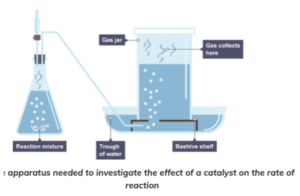
- Connect the flask to a measuring cylinder. Use a capillary tube to connect the two. The measuring cylinder should be upside down in a container of water, as seen in the diagram below.





Still got a question? Leave a comment
Leave a comment