Nanoparticles (GCSE Chemistry)
Nanoparticles
Nanoparticles
- Nanoparticles are tiny particles which are between 1-100nm in size. Structures that are 1-100nm in size and are made of a few hundred atoms are known as nanoparticles. These are extremely small. To put it into context nanoparticles can be 100 times smaller than fine dust.
- Nanoparticles have a large surface area to volume ratio. When the size of a cube decreases by a factor of 10 the surface area to volume ratio increases by 10. As nanoparticles are extremely small, they have a large surface area to volume ratio compared to other materials in the same bulk.
- Nanoparticles are more reactive than normal sized particles. Nanoparticles are more reactive than normal sized particles this is because nanoparticles have a large surface area to volume ratio. This means that there are more atoms or molecules on the surface of the nanoparticle compared to a normal particle, therefore more opportunity for reactions to occur.
Comparing Particle Sizes
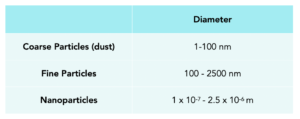
Particles can be categorised according to their size by using their diameter. Below is a table that can be used to categorise particles:





Still got a question? Leave a comment
Leave a comment