Filtration & Crystallisation (GCSE Chemistry)
Filtration & Crystallisation
Filtration
Filtration is used to separate liquids from insoluble solids. The liquid may have a soluble solute dissolved inside it.
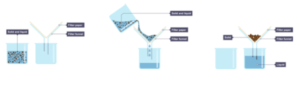
- We begin with an insoluble solid and a liquid. Let’s take a mixture of salt, sand and water. Salt dissolves in water, however the sand does not.
- Pour the mixture into the filter funnel. When you pour the mixture into a filter funnel lined with filter paper, the sand will be caught by the filter paper and the water with salt dissolved will drip through.
- The separation is complete. The liquid (salt solution) and solid (sand) are separated.
Crystallisation
Crystallisation is used to separate a soluble substance from a solvent. For example, crystallisation can be used to separate salt from a salt solution.
- We begin with a soluble solid dissolved in a solvent. We begin with a salt solution, containing salt (solute) dissolved in water (solvent).
- Warm the mixture in an evaporating basin. The mixture is poured into an evaporating basin and gently heated. This will evaporate away some of the solvent, which makes the solution more concentrated (i.e. more solute per unit solvent), until it becomes a saturated solution. This means that crystals start to develop. The point at which crystals form is called the point of crystallisation.
- Remove the heat. When insoluble crystals form, the heat is removed, and the saturated solution is allowed to cool. The crystals are separated from the remaining solvent using filtration and left to dry, within the folds of filter paper. The second step is key as it increases the concentration of the solution, hence making the crystals insoluble.
Filtration is a method to separate a solid and a liquid. The solid is collected on a filter paper, while the liquid passes through it and is collected in a separate container.
Filtration is used to remove solid impurities from liquids or to separate a solid from a liquid in order to obtain a pure substance.
Crystallisation is the process of forming solid crystals from a solution by allowing the solvent to evaporate slowly.
The steps involved in crystallisation are:
Dissolve a substance in a solvent to form a solution.
Cool the solution slowly to encourage the formation of crystals.
Allow the solvent to evaporate slowly, leaving behind the solid crystals.
Filter the solid crystals to obtain pure crystals.
Filtration is used to separate a solid and a liquid, while crystallisation is used to form solid crystals from a solution. Filtration is a physical process, while crystallisation is a chemical process.
Having pure substances is important in chemistry because impurities can affect the chemical reactions and the properties of the substance being used. By having pure substances, accurate and reliable results can be obtained in experiments.






Still got a question? Leave a comment
Leave a comment