Endothermic vs Exothermic Reactions (GCSE Chemistry)
Endothermic vs Exothermic Reactions
Energy
Transferring Energy
- Energy can be stored. Energy is stored in chemicals, with the amount of energy stored depending on the energy contained within the chemical bonds.
- Energy is conserved. During a chemical reaction, energy is conserved. This means that energy cannot be destroyed or created: it can only be exchanged with the surroundings when reactions occur.
- Energy is exchanged with the surroundings. When chemical reactions occur, through collisions, energy can be exchanged with the surroundings. If energy is released to the surroundings through a reaction, the product molecules must have less energy than the reactant. The difference in energy must equal the energy transferred.
Exothermic Reactions
- Energy is exchanged with the surroundings. During an exothermic reaction, energy is released to the surroundings.
- The temperature will rise. During an exothermic reaction, the temperature of the surroundings will rise. This is due to the energy being transferred to the surroundings.
Example Reactions
There are several reactions that release energy to the surroundings, therefore being classified as exothermic. These reactions include:
- Redox reactions including displacement reactions. All displacement reactions are types of redox reactions. Redox reactions involve both oxidation and reduction. For example, when iron oxide is reduced by carbon, energy is given out in the reaction.
Iron oxide + carbon → Iron + carbon dioxide
2Fe2O3(s) + 3C(s) → 4Fe(s) + 3CO2(g)
- Combustion or burning. For example, when magnesium is burnt in oxygen, a very bright light is observed and a great deal of energy is given out.
Magnesium + oxygen → magnesium oxide
2Mg (s) + O2(g) → 2MgO(s)
Burning fuels, like methane and petrol, release a great deal of useful energy.
- Neutralisation – acid + base reactions. When an acid is neutralised by a base or an alkali, energy is released.
For example:
Hydrochloric acid + sodium hydroxide → sodium chloride + water
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Specific Examples
- Self heating cans use exothermic reactions. Another example of everyday exothermic reactions comes in the form of self-heating drinks cans. Within the base of the can, exothermic reactions take place, releasing energy into the surroundings. The energy released is used to heat up the drink.
- Hand warmers oxidise iron. In order to heat up, hand warmers oxidise iron in air. This reaction releases energy to the surroundings and therefore can be classed as an exothermic reaction too.
Endothermic Reactions
- Energy is exchanged with the surroundings. During an endothermic reaction, energy is absorbed (or taken in) from the surroundings.
- The temperature will fall. During an endothermic reaction, the temperature of the surroundings will fall. This is due to the energy being taken in from the surroundings.
Example Reactions
There are a couple of reactions that absorb energy from the surroundings, therefore being classified as endothermic. These reactions include:
- Citric acid reacting with sodium hydrogencarbonate. Bubbles of carbon dioxide gas are formed and the temperature decreases during the reaction.
- Calcium carbonate can be thermally decomposed. If we heat up calcium carbonate, this causes it to thermally decompose into carbon dioxide and calcium oxide. The calcium oxide is sometimes known as quicklime. The reaction is endothermic, since it takes in heat from the surroundings.
CaCO3 + HEAT → CO2 + CaO
- Sports injury packs are endothermic. Instead of needing to be frozen, sports injury packs can undergo an endothermic chemical reaction. The reaction causes the pack to become cool instantly, by taking in energy from the surroundings.
Summary of Endothermic and Exothermic Reactions
- Temperature changes can occur. During both endothermic and exothermic reactions, temperature changes can occur. In endothermic reactions, the temperature of the surroundings will fall. In exothermic reactions, the temperature of the surroundings will rise.
- Energy can be exchanged. During both endothermic and exothermic reactions, energy is exchanged with the surroundings. Endothermic reactions absorb energy from the surroundings. Exothermic reactions will release energy to the surroundings.
Required Practical: investigating temperature changes of neutralisation reactions
For exams, you need to carry out experiments to investigate variables that can affect temperature changes in reacting solutions such as acid plus metals, acids plus carbonates, neutralisations and displacement of metals.
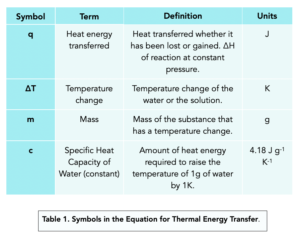
- We need to measure the amount of energy. During the experiment, we’ll be measuring the amount of energy released or absorbed during the reaction.
- A closed system is required. To get accurate results, we need to perform the experiment in a closed system. Our closed system will consist of a polystyrene cup with a lid, a beaker. The temperature is measured using a thermometer and reactants are placed into the cup.
For this required practical in particular, it is important to be aware of the variables involved.
- Dependent variable. Temperature change is the dependent variable, not temperature, as it is the change in temperature which is being measured.
- Independent variable. The independent variable is the amount of acid being added, as this is being changed in the investigation.
- Control variable. The control variables are the volumes in cm3 and concentrations in mol/dm3 of acid an alkali used. A polystyrene cup is used to control the heat loss from the reaction, as it is a good insulator.
Method
- Set up the equipment.Take two beakers, filling one with 25cm3 of 0.5mol/dm3 hydrochloric acid and the other with 40 cm3of the same concentration of sodium hydroxide.
- Insulate the cup. Place a polystyrene cup in a beaker. Polystyrene is used because it will insulate the solution and prevent energy losses to the surroundings in the form of heat.
- Combine the two liquids. Place a thermometer into the cup. Add all the hydrochloric acid into the polystyrene cup, and record the initial temperature. Then, using a 5cm3 measuring cylinder, add 4 cm3 of the sodium hydroxide and stir continuously.
- Record the temperature. Record the highest temperature reached.
- Repeat the experiment. Repeat the experiment, adding another 4cm3 of sodium hydroxide into the same solution, recording the highest temperature increase, until all the sodium hydroxide has been used up.
- Plot a graph. This is of the temperature recorded against the volume of sodium hydroxide added. Draw two straight lines of best fit on the graph
Worked example:
The results show the temperature from investigating the temperature change during a neutralisation reaction when small, equal volumes of sodium hydroxide solution is added to a solution of hydrochloric acid.
Plot the data and draw two straight lines of best fit and extrapolate them until they meet. (4 marks)
Determine the volume of sodium hydroxide needed to neutralise the acid. (2 marks)
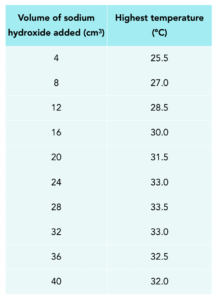
Answer:
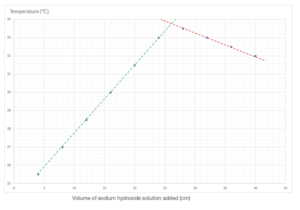
The point of neutralisation is the volume of sodium hydroxide which produces the highest temperature difference. It is where the two lines intersect. It is about 26.2 cm3.
Enthalpy Changes
Calculating the Enthalpy Change
We can calculate the amount of energy that is stored in or released from a system using this equation:
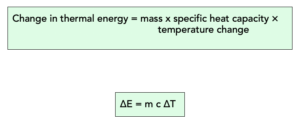
Where:
- change in thermal energy, ∆E, in joules, J
- mass, m, in kilograms, kg
- specific heat capacity, c, in joules per kilogram per degree Celsius, J/kg°C
- temperature change, ∆T, in degrees Celsius, °C
We cannot just measure the rise in temperature to find out the change in thermal energy, because the amount of heat energy needed to increase the temperature by 1°C varies dependent on the mass and type of liquid:
- The mass is accounted for in the equation
- The type of liquid is accounted for in the specific heat capacity. We will look at this below.
Practice question: Louisa heats up a 20kg block of concrete. She waits until the temperature has risen from 20 °C to 65 °C. Find the energy used to heat up the block, given that the specific heat capacity of concrete is 3200 J/kg°C.
1. We need to write out the equation for specific heat capacity.
∆E = m c ∆T
2. We need to calculate the temperature change.
We have not been told this value in the question, so we need to work it out.
65 – 20 = 45
3. We can use the formula.
We have all the numbers that we need, so we can simply substitute them into the formula. When we give our final answer, it may be more appropriate to give the units in terms of kilojoules.
∆E = m c ∆T
∆E = 20 x 45 x 3200 = 2 880 000 Joules
∆E = 2880 kJ
Molar Enthalpy Change
∆H represents the amount of energy lost or gained during a reaction. It is measured in kJ/mol.
- +∆H represents an endothermic reaction. The reaction uses heat from the surroundings.
- -∆H represents an exothermic reaction. The reaction loses heat to the surroundings.
We can use data from a calorimetry experiment, as seen above, to calculate the molar enthalpy change of a reaction.
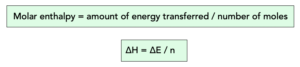
Where:
- change in thermal energy, ∆E, in joules, J
- molar enthalpy, ∆H, in kilojoules per mole, kJ/mol
- number of moles, n, in moles, mol
Practice question: In a combustion calorimetry experiment, 0.72g of ethanol (C2H5OH) produced 12265J of heat energy. Calculate the molar enthalpy change from the heat energy change. Give your answer to 2 dp.
1. We need to calculate the amount of ethanol burnt.
We need to use our question involving the mass and Mr to work out the amount of ethanol in moles.
Amount = mass / Mr
Amount = 0.72 / 46
Amount = 0.016 mol
2. Calculate the molar enthalpy.
Use the formula we learnt above to work out the molar enthalpy in kJ/mol, giving your answer to 2 decimal places.
∆H = ∆E / n
∆H = 12.265 / 0.016
∆H = 766.56 kJ/mol
∆H = – 766.56 kJ/mol
FAQs
An endothermic reaction is a chemical reaction that absorbs energy from its surroundings. This type of reaction results in a net gain of energy, which can result in an increase in temperature.
An exothermic reaction is a chemical reaction that releases energy into its surroundings. This type of reaction results in a net loss of energy, which can result in a decrease in temperature.
The main difference between endothermic and exothermic reactions is the direction of energy transfer. Endothermic reactions absorb energy from their surroundings, while exothermic reactions release energy into their surroundings.
Examples of endothermic reactions include the dissolution of ammonium nitrate in water, the reaction between baking soda and vinegar, and the reaction between citric acid and baking soda.
Examples of exothermic reactions include the combustion of gasoline, the reaction between baking soda and citric acid, and the reaction between magnesium metal and hydrochloric acid.
Endothermic and exothermic reactions can be identified by the direction of energy transfer. In an endothermic reaction, the temperature of the system decreases, while in an exothermic reaction, the temperature of the system increases.
Endothermic and exothermic reactions have a wide range of applications in various fields, including energy production, food and drink production, and pharmaceuticals. Endothermic reactions are used in refrigeration and air conditioning systems, while exothermic reactions are used in power generation and fuel production.
It is important to understand endothermic and exothermic reactions because they play a significant role in many natural and human-made processes. By understanding the concepts of endothermic and exothermic reactions, students can gain a deeper understanding of the energy changes that occur in chemical reactions, which is a fundamental concept in chemistry.





Still got a question? Leave a comment
Leave a comment