Using Concentrations of Solutions in mol/dm3 (GCSE Chemistry)
Concentration of Solutions Using Moles
As we learnt earlier, concentration can be calculated using the mass of a substance. The concentration of a solution can also be measured as the number of moles per unit volume. The units in this case are mol/dm3.
Calculating Mass from Concentration
We can use the following equation to calculate concentration using moles and volume.

The units of concentration are mol/dm3. This is because moles have the unit ‘mol’, and volume is measured in ‘dm3’.
Practice Question: Harry has 250 cm3 solution in a beaker containing 4 moles of calcium carbonate. Calculate the concentration of the solution.
1.Write out the equation.
Concentration = Moles / Volume
2. Convert into the correct units.
Volume must be in dm3.
Divide by 1000 to go from cm3 to dm3
250/1000 = 0.25 dm3.
3. Substitute in the numbers.
Concentration = moles / volume
Concentration = 4/0.25
Concentration = 16 mol/dm3
Using Concentration in Calculations
In these questions, we can use the ‘concentration = moles/volume’ formula and the tables which we use for mass and mole equations.
Practice Question: Jenny performed an experiment and found that 31.0cm3 of potassium hydroxide solution neutralised 25.0cm3 of dilute nitric acid, with a concentration of 2.0 mol/dm3. Calculate the concentration of the potassium hydroxide solution in moles per dm3.
1.Write out a balanced equation.
KOH + HNO3 -> KNO3 + H2O
2. Draw out a table.
3. Fill in the table with known values.
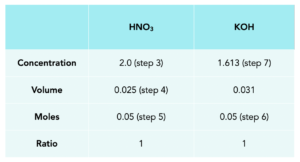
4. Convert the volumes into dm3.
Divide by 1000
1.Calculate moles of HNO3.
moles = concentration x volume
2. Calculate the moles for KOH.
3. Calculate the concentration of KOH in mol/dm3.
Concentration = moles/volume
Concentration = 1.61 mol/dm3.
FAQs
A concentration of a solution in mol/dm3 is a measure of the number of moles of solute (the substance being dissolved) present in one cubic decimeter of solution. It is a way to describe the amount of solute in a solution and is a commonly used unit in chemistry.
To find the concentration of a solution in mol dm^-3 (moles per cubic decimeter), you need to know the number of moles of solute and the volume of the solution in cubic decimeters (dm^3).
The formula to calculate the concentration (C) of a solution in mol dm^-3 is:
C = number of moles of solute (n) / volume of solution in dm^3 (V)
or
C = n / V
To calculate the number of moles of solute, you can use the formula:
n = mass of solute (m) / molar mass of solute (M)
or
n = m / M
where M is the molar mass of the solute in g mol^-1.
Once you have calculated the number of moles of solute and the volume of the solution in dm^3, you can substitute these values into the concentration formula to find the concentration of the solution in mol dm^-3.
Knowing the concentration of a solution in mol/dm3 is important because it allows for precise measurements and comparisons of the amount of solute in different solutions. This information is crucial in many chemical reactions and processes, as the concentration of a solution can affect the rate of reaction and the outcome of the reaction.
The concentration of a solution in mol/dm3 is calculated by dividing the number of moles of solute in the solution by the volume of the solution in cubic decimeters. The formula for this calculation is:
Concentration (mol/dm3) = Number of moles of solute / Volume of solution (dm3)
To prepare a solution with a specific concentration in mol/dm3, you need to know the desired concentration and the volume of solution that you want to prepare. You can then calculate the amount of solute needed using the formula:
Number of moles of solute = Concentration (mol/dm3) x Volume of solution (dm3)
Yes, the concentration of a solution in mol/dm3 can be converted to other units, such as g/dm3 or g/L, by using the molar mass of the solute. The molar mass can be used to convert the number of moles of solute to grams, which can then be used to calculate the concentration in g/dm3 or g/L.
Mol/dm3 and g/dm3 are different units that describe the concentration of a solution. Mol/dm3 is a measure of the number of moles of solute in a solution, while g/dm3 is a measure of the mass of solute in a solution. To convert from mol/dm3 to g/dm3, you need to know the molar mass of the solute and multiply it by the number of moles of solute.
In chemical reactions, the concentration of a solution in mol/dm3 is used to determine the amount of reactants needed to reach a certain reaction outcome. The concentration can also be used to determine the rate of reaction, as the concentration of reactants can affect the speed at which the reaction occurs. By manipulating the concentration of the reactants, it is possible to control the rate and outcome of a chemical reaction.
To calculate the mass of a solute from its concentration and volume, you need to know the concentration of the solution in mol dm^-3, the volume of the solution in dm^3, and the molar mass of the solute.
The formula to calculate the mass (m) of a solute from its concentration (C) and volume (V) is:
m = C x V x M
where M is the molar mass of the solute in g mol^-1.






Still got a question? Leave a comment
Leave a comment