Identifying Ions (GCSE Chemistry)
Identifying Ions
Identifying Ions is a fundamental skill in chemistry, crucial for understanding chemical reactions and compositions. This knowledge is directly applicable and highly valuable in various scientific fields, including when undertaking Chemistry work experience.
Flame Tests For Metal Ions
When metal ions are placed into a flame, they burn. When they burn, they produce a characteristic colour.
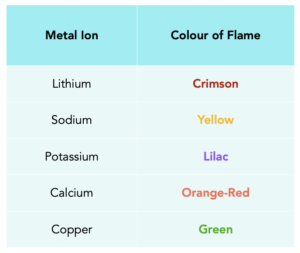
Flame Colours
For exams, you need to know the different colours produced when each of the following metal ions burn in a flame. They are shown in the table below.
It is worth noting that if there is a combination of ions in a mixture, then flame colour may be masked, or not clear.
Identifying Cations
- Sodium hydroxide identifies metal ions. In the test to identify metal ions, sodium hydroxide is used. More specifically, the metal ions that can be identified are cations.
- Coloured precipitates are formed. When the cations react with hydroxide ions, coloured precipitates are formed. We’ll look at each cation in more detail within this section.
Aluminium, Calcium and Magnesium Ions
- Sodium hydroxide forms a white precipitate. When sodium hydroxide is added to aluminium, calcium or magnesium ions, a white precipitate is formed.
- Sodium hydroxide can be added in excess. When sodium hydroxide is added in excess, the precipitate formed by aluminium hydroxide will dissolve. This does not happen with calcium or magnesium hydroxide.
Copper (II), Iron (II) and Iron (III) Ions
- Sodium hydroxide can form different coloured precipitates. When sodium hydroxide is added various coloured precipitates can be formed. A blue precipitate is formed by copper hydroxide, a green precipitate by iron (II) hydroxide and a brown precipitate by iron (III) hydroxide.
Nitrates and Sulphites
- Nitrate ions can be reduced to ammonia. Sodium hydroxide solution and aluminium powder can be added to a nitrate. By heating strongly, ammonia gas will be produced in the presence of nitrate ions. This can be identified by it’s smell or with litmus paper as mentioned in the previous tutorial.
- Sulphite ions can be detected by the presence of sulphur dioxide. By reacting a sulphite with a dilute acid, sulphur dioxide will be produced. Sulphur dioxide gas can be identified as mentioned in the previous tutorial.
Writing Balanced Equations
For exams, you will need to be able to write balanced equations for the reactions that we have just described. All the reactions produce insoluble hydroxides, which are all different colours.
Al3+(aq) +2OH–(aq) → Al(OH)3(s)
White precipitate soluble in excess sodium hydroxide solution
Ca2+(aq)+2OH–(aq) →Ca(OH)2(s)
White precipitate insoluble in excess sodium hydroxide solution
Mg2+ (aq)+2OH–(aq)→ Mg(OH)2(s)
White precipitate soluble in excess sodium hydroxide solution
Cu2+(aq) + 2OH–(aq)→ Cu(OH)2(s)
Blue precipitate soluble in excess sodium hydroxide solution
Fe2+ (aq)+2OH– (aq)→ Fe(OH)2(s)
Green precipitate soluble in excess sodium hydroxide solution
Fe3+ (aq)+2OH– (aq)→ Fe(OH)3(s)
Brown precipitate soluble in excess sodium hydroxide solution
Ions in GCSE Chemistry are charged particles that have either gained or lost electrons, making them either positively charged (cations) or negatively charged (anions).
Ions are formed in GCSE Chemistry when an atom gains or loses electrons, changing its electron configuration and resulting in a charged particle. This can occur through chemical reactions, such as ionization or oxidation, or through exposure to high-energy radiation.
Identifying ions in GCSE Chemistry is important because it allows scientists to understand the behavior of charged particles in chemical reactions and to predict the outcomes of these reactions. Ions play a critical role in many biological processes and in the formation of compounds and minerals.
Ions can be identified in GCSE Chemistry through a variety of methods, including:
Conductivity testing: Ions can be identified based on their ability to conduct electricity.
Flame testing: Ions can be identified based on the characteristic color they produce when burned in a flame.
Spectroscopy: Ions can be identified based on the specific frequencies of light they absorb or emit.
Precipitation reactions: Ions can be identified based on their ability to react with other ions to form a solid precipitate.
Some common ions that are studied in GCSE Chemistry include:
Sodium ion (Na+)
Chloride ion (Cl-)
Calcium ion (Ca2+)
Sulfate ion (SO42-)
Nitrate ion (NO3-)
The real-world applications of identifying ions in GCSE Chemistry include:
Water purification: The identification of ions is used in water purification to determine the presence of harmful contaminants, such as heavy metals, and to design effective treatment methods.
Medical diagnosis: The identification of ions is used in medical diagnosis to determine the presence of specific ions in bodily fluids, such as blood and urine, to diagnose diseases and monitor treatment.
Environmental monitoring: The identification of ions is used in environmental monitoring to determine the presence of pollutants in soil and water, and to monitor their effects on the environment.
Mining and minerals processing: The identification of ions is used in the mining and minerals processing industries to determine the presence of valuable minerals and to design effective extraction and processing methods.





Still got a question? Leave a comment
Leave a comment