The Current Periodic Table (GCSE Chemistry)
The Current Periodic Table
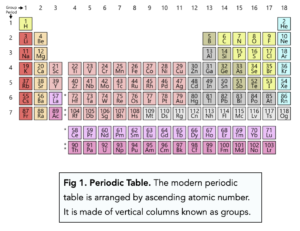
The Modern Periodic Table
- Discovery of isotopes of the same element explained the order based on atomic mass was not always correct. When scientists discovered isotopes it explained why some atoms had heavier atomic masses than expected. This meant that Mendeleev was correct to not stick to arranging the periodic table according to atomic mass.
- Elements in the modern periodic table are arranged according to atomic number. In the 20th century, protons and electrons were discovered by scientists who then realised the periodic table should be arranged according to atomic number rather than atomic mass.
- Order of elements in modern periodic table. Elements in the modern periodic table are arranged according to atomic number. The periodic table we use today is arranged according to the number of protons an element has also known as the atomic number. Before the 20th century, scientists had not discovered subatomic particles such as protons and electrons. Therefore scientists arranged elements in order of atomic weight in the past before this discovery was made.
Arrangement of the Periodic Table
- Elements with similar properties are arranged in vertical columns known as groups. In the periodic table, elements are arranged in vertical columns’; these are known as groups.
- Each group of elements has similar chemical and physical characteristics. Let’s take group 1 all the elements. in group 1 such as lithium, sodium and potassium are all very reactive alkali metals.
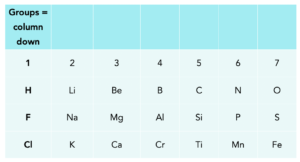
- Elements in the same group have the same number of electrons in their outermost shell. Elements in each group in the periodic table have a distinctive set of properties and react in the same way, this is due to the number of electrons in the outermost shell. When atoms of elements react, they either gain, lose or share the electrons in their outermost shell.
- Elements in the periodic table are also arranged in horizontal rows. The periodic table is formed of multiple rows, these are known as periods. The elements are arranged in periods in ascending atomic numbers. Each period signifies the number of shells that the atoms of the elements in that period have. For example, lithium is in the second period and has 2 shells.
- Group Number. The group number at the top signifies the number of electrons in the outermost shell.
- Period.There are also horizontal rows known as periods. These rows tell you the number of electron shells that an atom has.





Still got a question? Leave a comment
Leave a comment