Size & Mass of Atoms (GCSE Chemistry)
Size & Mass of Atoms
Size of an Atom
- Atoms are extremely small. Each individual atom is incredibly small and can not be seen by the human eye. The radius of each atom is around 0.1nm, which in standard form is 1 x 10-10 m.
- The nucleus is only a tiny part of an atom. Each atom has a central nucleus and the radius of this nucleus is around 1 x 10-14 m. The nucleus is tiny compared to the whole atom. The radius of the nucleus is less than 1/10,000 of the radius of the whole atom. This means that most of the atom is occupied by the cloud of electrons.
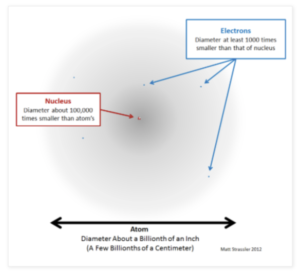
Fig X. Size of an atom. The central nucleus (red) is extremely small compared to the size of Rutherford’s nuclear model. Positive charge is found in the centre (red) and electrons are found around (blue). (https://dispatchesfromturtleisland.blogspot.com/2013/04/an-atom-drawn-to-scale.html)
Mass Number
The mass number is the total number of protons and neutrons which are found in the nucleus. Electrons have a negligible mass, so we don’t usually count it when working out the mass of an atom.
- Protons, neutrons, electrons have a relative mass. Both protons and neutrons have a relative mass of 1. Electrons have a really small mass so it is usually assumed as 0.

- The majority of the mass of an atom is in the nucleus. The nucleus consists of the majority of the mass of an atom because the subatomic particles within the nucleus (the protons and the neutrons) both have a relative mass of 1. Whereas the electrons surrounding the nucleus have a very small mass; the mass is negligible.
Calculations of Protons, Neutrons, Electrons
We can perform calculations to determine the number of protons, neutrons and electrons within an atom:
Worked Example: Use your periodic table to calculate the number of protons, neutrons and electrons in phosphorus.
Answer:
- Find phosphorus on the periodic table.
It has a mass number of 31 and an atomic number of 15
- Number of protons = number of electrons.
Protons = 15, therefore electrons = 15
- Number of neutrons = Atomic mass number – Atomic number
31-15= 16 neutrons





Still got a question? Leave a comment
Leave a comment