Addition Polymerisation (GCSE Chemistry)
Addition Polymerisation
Polymerisation Reactions
- Polymers are formed from repeating units. Polymers are molecules that are made up of much smaller molecules. The smaller molecules are called monomers.
- Polymerisation reactions form polymers. Polymers are made via polymerisation reactions. This is the process by which the monomers can join together to form polymers.
- Specific conditions are required. Sometimes, specific conditions are required for polymerisation reactions to occur. For example, a high pressure and presence of a catalyst may be required.
Addition Polymerisation
- Addition polymerisation is a type of polymerisation. In addition polymerisation, is one type of polymerisation. Many molecules called monomers will join together to form polymers. This is possible, because all monomers involved in addition polymerisation have a double bond between the carbon atoms.
- One monomer is repeated. In the process of addition polymerisation, only one monomer is used as the repeating unit. The repeating unit of the polymer will have the same atoms as the monomer. To show that many monomers are involved, an “n” is placed before the monomer.
- One polymer is produced. At the end of the addition polymerisation reaction, only one product is formed. This product is the polymer chain. An example product would be poly(ethene), formed by lots of ethene monomers.
- There are many common addition polymers. Other examples include propene, which polymerises to poly(propene) or polypropylene or chloroethane which polymerises to poly(chloroethene), also known as PVC.
Representing Addition Polymers
- Addition polymers can be drawn out. One of the easiest ways to represent addition polymers and their monomers is to draw them out, similar to displayed formulae.
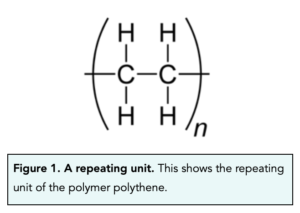
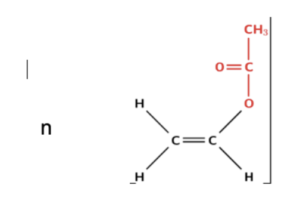
- Brackets are used to represent monomers. When representing monomers, brackets are placed either side of the ‘free’ carbon bonds. This signifies where the other carbon atoms in the polymer would bind. Below, we can see the repeating unit for polythene.
- The ’n’ shows the number of monomers. The small ’n’ after the brackets represents the number of monomers that form a polymer. This could also be described as the number of repeating units in the polymer.
- The bonds from the C atoms extend beyond the brackets. To show that this is a repeating unit and the molecule extends beyond this, the bonds from the C atoms must extend beyond the brackets on both ends of the repeating unit.
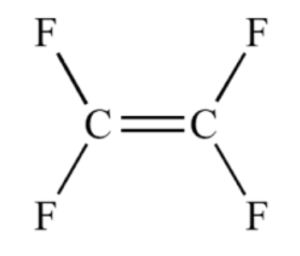
Worked example: Draw the repeating unit from the monomer, tetrafluoroethene, shown here.
Answer:
- Open out the double bond and replace this with a single bond.
- Draw a bond on the other side of each carbon atom to show the double bond has opened up.
- Add the groups/elements that are attached to each carbon atom, exactly as the appear in the monomer.
- Draw brackets around the repeating unit, ensuring the bonds extend beyond the brackets.
- Place an “n” as a subscript on the lower right hand side of the repeating unit
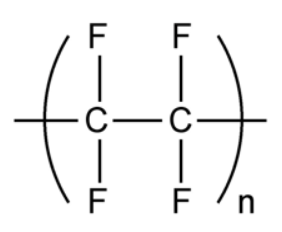
Worked example: Draw the monomer from the repeating unit shown here.
Answer:
- Draw all the atoms in the same places they occur, above and below the C atoms, in the repeating unit.
- Draw bonds in the side groups exactly as they appear in the repeating unit.
- Draw single bonds from each carbon atom to the atoms above and below.
- Draw a double bond between each carbon atom.
- Add an “n” before the monomer to show there are many units adding together
Addition polymerization is a process in which monomers, which are small organic molecules, are chemically bonded together to form a polymer, which is a long chain of repeating units.
Addition polymerization occurs when monomers are joined together through a chemical reaction that involves the removal of a small molecule, such as a hydrogen atom or a molecule of water. This reaction is facilitated by a catalyst, which speeds up the reaction. The resulting polymer is a chain of repeating units that is much larger than the original monomers.
Key features of addition polymerization include the formation of a long chain of repeating units, the removal of a small molecule during the reaction, and the use of a catalyst to speed up the reaction.
Applications of addition polymerization include the production of plastics, fibers, and adhesives, as well as the production of synthetic rubber. Additionally, addition polymerization is used to produce certain medical products, such as artificial joints and implants.
Advantages of addition polymerization include the ability to produce large amounts of polymer at a low cost, the ability to control the properties of the polymer through the selection of monomers and catalysts, and the ability to produce polymers with a wide range of properties. Disadvantages of addition polymerization include the production of waste and the potential for the release of harmful chemicals into the environment.
The structure of a polymer, including its molecular weight, the arrangement of its repeating units, and the presence of impurities, affects its properties. For example, a polymer with a high molecular weight will have different mechanical properties than a polymer with a low molecular weight. Additionally, the presence of impurities can affect the polymer’s stability, strength, and other properties.





Still got a question? Leave a comment
Leave a comment