Covalent Bond Diagrams (GCSE Chemistry)
Covalent Bond Diagrams
Representing Covalent Bonding
Dot and Cross Diagram
- Dot and cross diagrams can be used to illustrate covalent bonding. A dot and cross diagram can show electrons being shared between two non-metals where electrons from one atom are shown as dots and electrons from another atom as crosses.
- Structural diagrams can be used to illustrate covalent bonding. Each pair of shared electrons can be represented by a single line “ – “ to join the atoms.
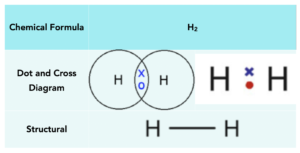
Hydrogen
Hydrogen atoms have 1 electron in their outer shell and require 1 more electron to have a full outer shell. To achieve a full outer shell, two hydrogen atoms form a covalent bond sharing a part of electrons. This leaves both hydrogen atoms with 2 outer electrons.
Below is a table that shows the manner in which hydrogen can be represented:
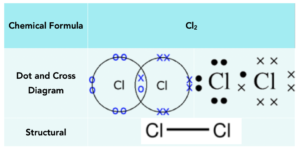
Chlorine
Chlorine atoms have 7 electrons in their outer shell and require 1 more electron to have a full outer shell. To achieve a full outer shell, two chlorine atoms form a covalent bond sharing a part of electrons. This leaves both chlorine atoms with 8 outer electrons.
Below is a table that shows the manner in which chlorine can be represented:
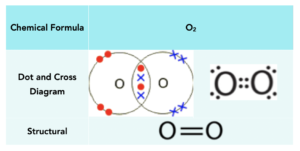
Oxygen
Oxygen atoms have 6 electrons in their outer shell and require 2 more electrons to have a full outer shell. To achieve a full outer shell, two oxygen atoms share two pairs of electrons with each other forming a double covalent bond. This leaves both oxygen atoms with 8 outer electrons.
Below is a table that shows the manner in which oxygen can be represented:
Nitrogen
Nitrogen atoms have 5 electrons in their outer shell and require 3 more electrons to have a full outer shell. To achieve a full outer shell, two nitrogen atom share three pairs of electrons with each other forming a triple covalent bond. This leaves both nitrogen atoms with 8 outer electrons.
Below is a table that shows the manner in which hydrogen can be represented:
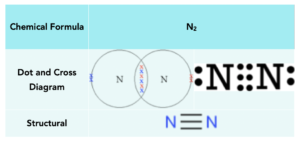
Hydrogen chloride
Hydrogen and Chlorine both need to gain 1 electron to have a full outer shell. So they can form one covalent bond in HCl to achieve full outer shells.
Below is a table that shows the manner in which hydrogen chloride can be represented:
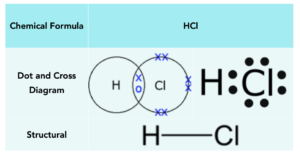
Water
Oxygen atoms need 2 electrons to form a full outer shell, whilst hydrogen atoms need 1 electron. Therefore, hydrogen atoms need to form one covalent bond to get a full outer shell, and oxygen needs to form two covalent bonds. Therefore we can take 2 hydrogens and 1 oxygen, forming 2 covalent bonds in total. This leaves the oxygen with 8 outer electrons.
Below is a table that shows the manner in which water can be represented:
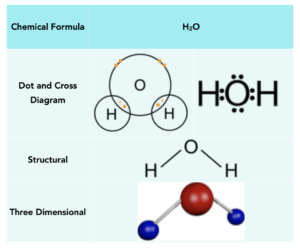
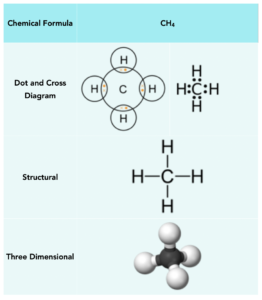
Methane (CH4)
Carbon atoms need 4 electrons to form a full outer shell, whilst hydrogen atoms need 1 electron. Therefore, hydrogen atoms need to form one covalent bond to get a full outer shell, and carbon needs to form four covalent bonds. Therefore we can take 4 hydrogens and 1 carbon, forming 4 covalent bonds in total. This leaves the carbon with 8 outer electrons.
Below is a table that shows the manner in which methane can be represented:
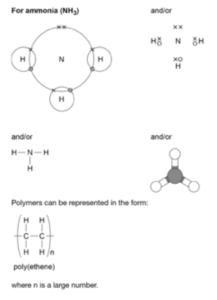
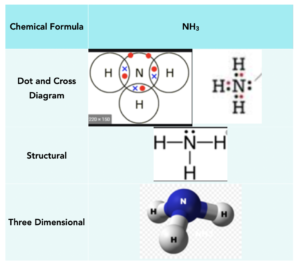
Ammonia (NH3)
Nitrogen is in group 5, and therefore needs 3 electrons to form a full outer shell. Hydrogen atoms need 1 electron. Therefore, hydrogen atoms need to form one covalent bond to get a full outer shell, and nitrogen needs to form three covalent bonds. Therefore we can take 3 hydrogens and 1 nitrogen, forming 3 covalent bonds in total. This leaves the nitrogen with two unbonded electrons on the outer shell.
Below is a table that shows the manner in which methane can be represented:
FAQs
A covalent bond is a type of chemical bond where two or more atoms share electrons in order to form a molecule. This sharing of electrons creates a strong bond between the atoms, which holds them together in a molecule.
Covalent bonds are represented in diagrams using a line between the atoms that are bonded. The number of lines in the diagram represents the number of bonds between the atoms.
The dots in a covalent bond diagram represent the valence electrons (the outermost electrons) of an atom. In a covalent bond, atoms share valence electrons in order to form a bond.
The number of covalent bonds an atom can form depends on the number of valence electrons the atom has. Typically, atoms can form up to four covalent bonds.
The difference between single, double, and triple covalent bonds is the number of electrons that are shared between the atoms.
A single covalent bond involves the sharing of two electrons between the atoms.
A double covalent bond involves the sharing of four electrons between the atoms.
A triple covalent bond involves the sharing of six electrons between the atoms.
Understanding covalent bond diagrams is important because it helps us to understand the structure of molecules and how they are held together. This information is useful in various fields, such as medicine, engineering, and environmental science, where it is important to understand the properties and behavior of molecules. Additionally, understanding covalent bonds can help in the design of new materials, the development of new medicines, and the solving of environmental problems.
Yes, covalent bonds can be broken. Covalent bonds can be broken by adding energy to the molecule, for example, by heating it or applying a chemical reaction. When the bond is broken, the atoms become separate entities and can form new bonds with other atoms.
Covalent bonds have a significant impact on the properties of a substance, such as its boiling and melting points, solubility, and reactivity. For example, substances with strong covalent bonds tend to have higher boiling and melting points, while substances with weak covalent bonds tend to have lower boiling and melting points. Understanding the strength and nature of covalent bonds is important in determining the properties of a substance and how it will behave in various situations.






Still got a question? Leave a comment
Leave a comment