Atomic Models Part 1 (GCSE Chemistry)
Atomic Models Part 1
Models for Atoms
Models used to explain atoms have changed overtime as scientists gather new evidence through conducting experiments. We will first look at a summary of the first two stages in developing the model for an atom accepted today.
Billiard Ball Model (John Dalton)
In the early 1800s, John Dalton conducted many experiments, making the following findings to form the Billiard Ball model:
- Elements are made of tiny particles called atoms.
- Atoms were spherical in shape and could not be divided or split.
- During chemical reactions, atoms can be combined, separated or rearranged.
- Atoms were solid spheres.
Plum Pudding Model (Thompson)

In the late 1800s, Thompson discovered negatively charged particles within the atom, known as electrons. Here is a summary of his findings:
- Negatively charged electrons are present in atoms.
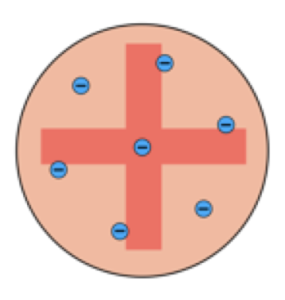
- He proposed the ‘plum pudding’ model, which suggested the atom was a positively charged sphere with negatively charged electrons randomly embedded within it.
- The mass of the atom is equally distributed throughout the atom.
Thompson believed that the atom (pudding) was a ball of positive charge, with electrons (plums) embedded within it.





Still got a question? Leave a comment
Leave a comment