Ionic Compounds (GCSE Chemistry)
Ionic Compounds
Ionic Structure
- Ionic compounds are held together by ionic bonding. In an ionic compound there are strong electrostatic forces of attraction between oppositely charged ions, this is known as ionic bonding. These forces operate in all directions holding the oppositely charged ions together.
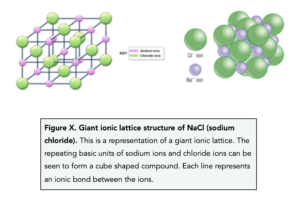
- Ionic compounds form crystals with a giant ionic lattice structure. A giant ionic lattice structure is a structure that has a regular arrangement of alternating positive and negative ions in a three dimensional shape.
Worked example: Explain the bonding in magnesium fluoride. (6 marks)
1. Work out how many electrons each atom has in the outer shell. Magnesium is in Group 2 and has 2 electrons in the outer shell. Fluorine is in group 7. It has 7 electrons in its outer shell.
2. Draw a dot and cross diagram. The diagram will show the transfer of electrons from the metal to the non metal.
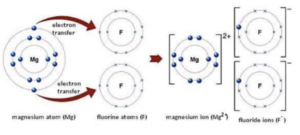
3. Explain how the ionic bond is formed. State the number of atoms and the number of electrons transferred.
One magnesium atom transfers one electron to two fluorine atoms ( 2 marks)
The magnesium atom becomes an Mg2+ ion
Each fluorine atom becomes an F– ion (1 mark)
The Mg2+ and F– ions attract (1), with strong electrostatic forces of attraction (1), acting in all directions to become a giant 3-D lattice (1).
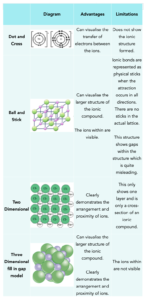
Representing Ionic Compounds
Ionic compounds can be represented through many modalities, including a few that we have covered thus far including the dot and cross diagram. Below is a table to outline the different modalities highlighting the pros and cons for each.
Empirical formula
The empirical formula is the simplest ratio of atoms or ions within a structure.
To work out the empirical formulas from a diagram, count the number of each ion and finally work out the lower common ratio.
Worked example: What is empirical formula of the ionic compound here:
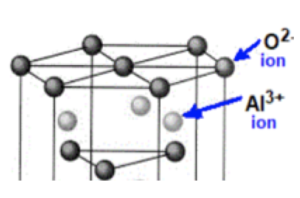
Answer:
Number of Al3+ ions in image = 3
Number of O2- ions in image = 10
So in this case, from the information given the the empirical formula is
Al₃O₁₀.
Naming Ionic Compounds
The ending of the name of an ionic compound can tell us how many elements are present.
If the name of the ionic compounds ends in:
- –ide, the compound contains only two elements. For example potassium iodide, KI.
- –ate, the compound contains three or more elements, one being oxygen. For example, potassium iodate, KIO₃.





Still got a question? Leave a comment
Leave a comment