Limiting Reactants (GCSE Chemistry)
Limiting Reactants
Limiting Reactants
- Limiting reactants are completely used up in a reaction, causing it to stop. Lots of reactions have limiting reactants. It’s common to add an excess of the other reactants, just to make sure that the limiting reactant is completely used up in the reaction. The limiting reactant is the reactant which is used up completely in a reaction.
- The amount of product formed is linked to the amount of limiting reactant. We can call the relationship between amount of limiting reactant and amount of limiting product a directly proportional relationship. For example if the limiting reactant is tripled, then the product formed is tripled. Similarly, if you halve the amount of limiting reactant then you will get half the product produced.
Calculating Product Formation Based on the Limiting Reactant
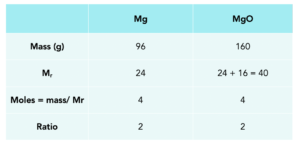
Practice Question: We burn 96 grams of magnesium in excess air. What mass of magnesium oxide is formed?
1.Write out a balanced equation. To calculate the mass, we firstly need to write out the balanced equation.
Magnesium + oxygen -> Magnesium oxide
2Mg + O2 -> 2MgO
2. Use the table. We can fill in the mass of Mg, which was stated in the question.
3. Work out the Mr. We can work out the Mr of both Mg and MgO
4. Calculate moles. We calculate the moles of Mg,
5. Use the balanced equation. We can work out the ratio of Mg:MgO from the balanced equation.
Put in the number of moles for MgO
6. Work out the mass of MgO. We can do this using the rearranged formula.
mass = moles x Mr
Mass of MgO formed is 160g.
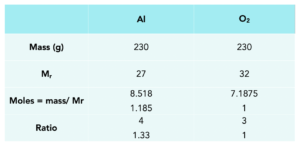
Worked example: 230 g of Al reacts with 230 g of oxygen. Determine which reactant is limiting and the mass of aluminium oxide produced.
1.Write out a balanced equation. To calculate the mass, we firstly need to write out the balanced equation.
Aluminium + oxygen → aluminium oxide
4Al(s) + 3O2(g) → 2Al2O3(s)
2. Use the table. We can fill in the mass of Al and O2, which was stated in the question.
3. Work out the Mr. We can work out the Mr of both Al and O2
4. Calculate moles. We calculate the moles of Al and O2.
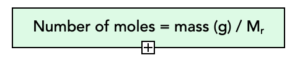
5. Use the balanced equation. We can work out the ratio of Al:O2 from the calculated moles. Divide by the smallest number of moles to find the calculated mole ratio.
6. Compare calculated mole ratio with mole ratio from equation. The substance with fewer moles compared to the balanced equation ratio, is limiting.
The calculated mole ratio of Al (1.185) is lower than that of the balanced symbol equation. This means that aluminium is the limiting reactant.
7. Work out the expected mass of product. Use the moles of limiting reactant We can do this using the rearranged formula.
Calculated moles of aluminium = 8.518 mol
Mole ratio of from balanced equation Al:Al2O3 = 4:2 = 2:1
Therefore moles of Al2O3 = 4.259 mol
mass = moles x Mr
Mr of Al2O3 = (27×2) + (16×3) = 102
Mass of Al2O3 formed is 4.259 x 102 = 434.42g
A limiting reactant is a reactant that determines the maximum amount of product that can be produced in a chemical reaction. This is because the limiting reactant is the one that is used up first and restricts the amount of product that can be produced.
To find the limiting reactant, you need to determine the number of moles of each reactant that is present in the reaction. Then, you need to calculate the number of moles of product that could be produced from each reactant. The reactant that produces the smallest amount of product is the limiting reactant.
Once the limiting reactant has been used up, the excess reactant remains in the reaction mixture. This excess reactant does not contribute to the formation of product and is typically wasted in the reaction.
The limiting reactant determines the amount of product that can be produced in a chemical reaction. This is because the limiting reactant restricts the amount of product that can be formed by limiting the amount of reactant that is available for the reaction to proceed.
To increase the amount of product produced in a reaction with a limiting reactant, you need to add more of the limiting reactant. By doing so, you increase the amount of reactant that is available for the reaction to proceed, which leads to an increase in the amount of product produced.
A limiting reactant is a reactant that restricts the amount of product that can be produced in a chemical reaction. An excess reactant is a reactant that is not completely used up in the reaction and remains in the reaction mixture. The excess reactant does not contribute to the formation of product.





Still got a question? Leave a comment
Leave a comment