Using Moles to Balance Equations (GCSE Chemistry)
Using Moles to Balance Equations
Using Reacting Masses to Balance Equations
If we have the value of the masses of each compound in an equation, we can work out how to balance an equation.
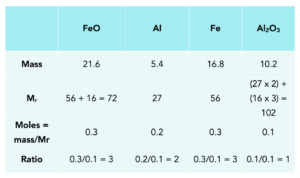
Practice Question: 21.6g of Iron oxide (FeO) reacts with 5.4g of Aluminium to form 16.8g of iron (Fe) and 10.2g of Aluminium Oxide (Al2O3). Write a balanced symbol equation for this reaction.
In this example, we return to the table that we’ve just seen to work out the multipliers.
- Place the known masses into the table for each of our substances. Here, we know the masses of iron oxide, aluminium, iron and aluminium oxide.
- Work out the Mr. Using our Periodic Tables, we can work out the Mr for each of the substances.
- Calculate the moles. For each of the substances in the equation, we can calculate the moles using our formula.
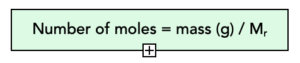
Smallest value are the moles of Al2O3 = 0.1 mol.
Therefore, divide all values by 0.1 mol to calculate the moles ratio for the equation.
3FeO + 2Al -> 3Fe + 1Al2O3.
Moles are a unit of measurement used in Chemistry to quantify the amount of a substance. It is the number of particles (atoms, molecules, or ions) in a substance, and is represented by the symbol “mol.”
Balancing chemical equations involves making sure that there is the same number of atoms of each element on both sides of the equation. Moles are used to determine the number of particles present, so that the coefficients of the equation can be adjusted to ensure that the number of atoms is equal on both sides.
Balancing chemical equations is important because it represents a chemical reaction that occurs in nature. An unbalanced equation would not accurately represent the reaction and the results of any calculations based on the reaction would be incorrect.
Sure! Let’s consider the following chemical equation: H2 + O2 → H2O To balance this equation using moles, we first need to determine the number of atoms of each element present. In this case, there are 2 hydrogen atoms and 2 oxygen atoms on the reactant side, and 2 hydrogen atoms and 1 oxygen atom on the product side.
To balance the equation, we add a coefficient of 2 in front of the water (H2O) on the product side to get: H2 + O2 → 2H2O. Now, the number of atoms of each element is equal on both sides of the equation.
Once a chemical equation is balanced, the moles of a substance can be used to calculate the amount of another substance required in a reaction. This is because the ratio of the coefficients in a balanced equation represents the ratio of the number of moles of each substance present. For example, if we know the number of moles of one reactant, we can use the coefficients in the balanced equation to determine the number of moles of other reactants and products.
The mole concept is directly related to Avogadro’s number, which is defined as the number of particles in one mole of a substance. For example, one mole of a substance contains 6.022 x 10^23 particles. This number allows us to convert between the number of particles and the number of moles of a substance, which is essential in many chemical calculations.





Still got a question? Leave a comment
Leave a comment