Polymers (GCSE Chemistry)
Polymers
Polymers
- A polymer is a large molecule made up of repeating subunits known as monomers. Within a polymer, there are many identical small molecules known as monomers. These monomer molecules are covalently bonded together forming a long chain. Let’s take poly(ethene) as an example. This polymer is made of multiple repeating units of ethene (C2H4).
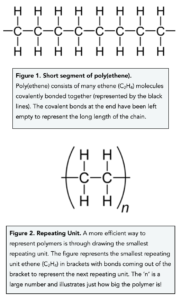
- Polymers are solid at room temperature. At room temperature polymers are solid, this is because between these long chains of polymers there are many weak intermolecular forces that make the overall intermolecular forces relatively strong. These intermolecular forces are more difficult to overcome and require more energy, therefore at room temperature these polymers are solid.
Polymers are long chains of repeating units called monomers, which are linked together through chemical bonds. They are important in chemistry because they have unique properties, such as high strength, flexibility, and resistance to heat and chemicals, making them useful in a wide range of applications, from packaging materials to medical devices.
The two main types of polymers are addition polymers and condensation polymers. Addition polymers are formed through the addition of monomers with unsaturated bonds, while condensation polymers are formed through the elimination of a small molecule, such as water, during the linking of monomers.
Polymerization is the process of linking monomers together to form a polymer. It can occur through addition polymerization or condensation polymerization, depending on the type of monomers used. In general, it involves the formation of strong covalent bonds between the monomers, resulting in a larger, more complex molecule.
Some common examples of polymers include plastics, such as polyethylene and polypropylene, synthetic fibers, such as nylon and polyester, natural polymers, such as cellulose and DNA, and biopolymers, such as proteins and carbohydrates.
Polymers have several advantages over traditional materials in industry, such as being lightweight, durable, and easily molded into various shapes and sizes. They are also often cheaper and more readily available than natural materials, making them an attractive option for mass production.
While polymers have many practical uses, their disposal can be an environmental concern, as they do not readily biodegrade and can persist in the environment for hundreds of years. This has led to concerns about plastic pollution and the need for more sustainable methods of polymer production and waste management.
Thermoplastics are polymers that can be melted and reformed multiple times, while thermosetting plastics are polymers that undergo irreversible chemical changes when heated, resulting in a rigid, heat-resistant material that cannot be melted or reshaped.
Crosslinking is the process of linking adjacent polymer chains through chemical bonds, resulting in a three-dimensional network structure. This can increase the strength, stiffness, and heat resistance of the polymer, making it useful in applications such as adhesives and coatings.
Polymers have numerous applications in medicine, such as in drug delivery systems, surgical implants, and wound dressings. They can be designed to be biocompatible, biodegradable, and/or bioactive, making them useful in a variety of medical contexts.





Still got a question? Leave a comment
Leave a comment