Soluble & Insoluble Salts (GCSE Chemistry)
Soluble & Insoluble Salts
What is a soluble salt?
A Soluble salt is a salt that can be easily dissolved in water. Read on for more information about making and preparing soluble salts.
Making Soluble Salts
What must be reacted together to produce a soluble salt?
Through a neutralisation reaction, we can produce soluble salts. In order to do this, we must react an acid with an insoluble base.
- We must use an insoluble base. In order to form a soluble salt, we must use an insoluble base. This could be a solid insoluble substance such as a metal, metal oxide, hydroxide or carbonate.
- The base is added to the acid. When we want to form a soluble salt, we must add an insoluble solid base to an acid. This is done until no more base will react with the acid. At this point, we can filter out the excess solid, leaving us with a solution of the salt.
- We can crystallise salt solutions. At the end of the experiment, we have obtained a solution containing the salt. In order to get the salt as a solid, we must crystallise the solution.
Preparing Soluble Salts
In this experiment, we are going to make a soluble salt using an insoluble base (it will not dissolve in water.) When we add the insoluble base to an acid, the two will react to form a soluble salt.
- Pick the acid and base. For this reaction, we need to be careful when choosing our acids and bases. Our choice depends on the product that we want to form. Remember, the base must be insoluble.
- Warm the acid. For this experiment, we will be using dilute acid, which we can warm up by using a Bunsen Burner. Once the acid is warm, we can start adding our insoluble base.
- Add the base in excess. We need to add the base to the warm acid until it is in excess. This is the point where no more reactions will occur: the insoluble base no longer dissolves in the acid.
- Filter the excess solid. To remove the excess base that has not reacted, we need to use a funnel with some filter paper. The solution that we filter will contain the soluble salt.
- Crystallise the solution. So that we get a pure sample of solid salt at the end of the reaction, we need to crystallise the solution. We can do this by heating up the solution gently, using a water bath, then leaving the solution to cool. This allows salt crystals to form, which can be filtered out and dried.
An example is the preparation of pure dry crystals of copper sulphate (CuSO4) from copper oxide (CuO) and sulphuric acid (H2SO4).
Solubility
General Rules of Solubility
Solubility is used to measure the maximum mass of a solute that can be dissolved in a given volume of a solvent. You need to know about the solubility rules of several common types of substances in water. This is summarised in the table below.
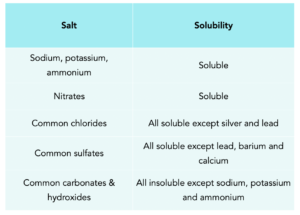
Predicting the Solubility
You need to know if a precipitate will form when different solutions are mixed together. This can be predicted using solubility rules, based on if the salt formed is soluble or insoluble in water, as shown in the table above.
A salt can be prepared by titration if soluble in water.
A salt can be prepared by precipitation if insoluble in water.
Preparing Insoluble Salts
In this experiment, we are going to make a pure, dry sample of an insoluble salt using a dilute acid and an insoluble base.
- Pick the acid and base. For this reaction, we need to be careful when choosing our acids and bases. Our choice depends on the product that we want to form. Remember, the base must be insoluble.
- Dissolve in water. Mix together using a stirring rod in a beaker.
- Filter the excess solid. To remove the excess base that has not reacted, we need to use a funnel with some filter paper.
- Wash the filtrate. Make sure you use deionised water to remove any other solutions present.
- Dry the insoluble salt. It can be left in an oven to dry allowing any water to evaporate.
An example is the preparation of a pure, dry sample of lead (II) sulphate (PbSO₄) from lead (II) nitrate solution (Pb(NO₃)₂) and sodium sulphate solution (Na₂SO₄).
Soluble salts are salts that dissolve in water to produce a solution with a homogeneous composition. Insoluble salts are salts that do not dissolve in water and remain as a solid in the solution.
Soluble and insoluble salts are important in GCSE chemistry because they provide a basic understanding of solubility and the behavior of substances in solution. This knowledge is essential for understanding many chemical reactions and processes.
The solubility of a salt refers to the maximum amount of a salt that can dissolve in a given amount of water at a specific temperature and pressure.
The solubility of a salt is usually influenced by temperature. In general, increasing the temperature of a solution will increase the solubility of a salt.
The solubility of a salt is generally not affected by pressure, except in the case of gases. Increasing the pressure on a gas in solution can increase its solubility.
Yes, a soluble salt can become insoluble. This can occur when a reaction between the salt and other substances in the solution takes place, causing the salt to precipitate out of solution.
Yes, an insoluble salt can become soluble by dissolving in a solvent other than water or by heating the solution to a high temperature.
Some common examples of soluble salts include sodium chloride, potassium nitrate, and calcium chloride.
Some common examples of insoluble salts include calcium carbonate, lead sulfate, and iron (III) hydroxide.





Still got a question? Leave a comment
Leave a comment