Changing State (GCSE Chemistry)
Changing State
Changing State is a fundamental concept in chemistry, illustrating how matter transitions between solid, liquid, and gaseous forms. Understanding these processes is not only crucial for academic success in GCSE Chemistry but also provides a foundational insight for various scientific fields, including potential Chemistry work experience in laboratories or industrial settings.
Changes of State
- In order to change the state of a substance, energy is required. To change state from a solid to a liquid and a liquid to a gas, energy needs to be put in to break the forces between the particles.
- The energy required to change state depends on the strength of the bonds holding the particles together. Different substances have different types of bonding and forces holding the substance together. The amount of energy required to change state is dependent on the type of bonding involved. For example, simple molecules have a lower melting and boiling point compared to ionic compounds.
- The stronger the force between particles the higher the melting and boiling point of the substance. The forces that hold particles together can be strong. Large amounts of energy are needed to overcome these forces, therefore a high temperature is required.
- Changing state is a physical change. During a physical change, substances change state, for example from a solid to a liquid. This is different to a chemical change, where new elements or compounds are formed in the reaction.
Melting and Boiling
- Melting and boiling points of ionic compounds. When melting and boiling ionic substances, the strong electrostatic forces of attraction are overcome. These forces require large amounts of energy to overcome. Therefore, ionic compounds have high melting and boiling points.
- Melting and boiling points of simple molecules. Between simple molecules, there are weak intermolecular forces. The intermolecular forces are easily overcome and require little energy to break. Therefore, simple molecules have lower melting and boiling points.
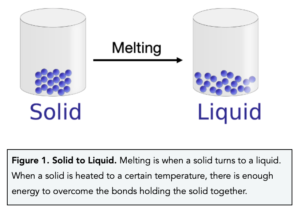
Melting
- Within a solid the particles are closely packed together in a regular arrangement.
- As a solid is heated, the particles gain more energy and start to vibrate.
- As the temperature increases further, the particles gain more the particles vibrate more vigorously.
- At a certain temperature, known as the melting point, there is enough energy to overcome the bonds holding the solid together.
- The particles then break out of the regular arrangement and start to move.
- A liquid is now formed.
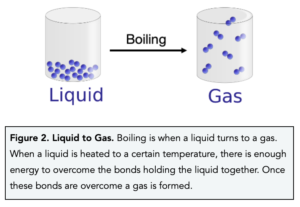
Boiling
- Within a liquid the particles are arranged in a random manner and are free to move.
- As a liquid is heated, the particles gain more energy and start to move around faster.
- As the temperature increases further, particles start to escape from the surface of the liquid this is known as evaporation.
- At a certain temperature, known as the boiling point, bubbles of gas form throughout the liquid.
- These bubbles come to the surface and a gas is formed.
Predicting States of Substances
When predicting states of substances at a given temperature it is important to compare this value to the melting and boiling point. Below is a scale that summarises this:
The circles below represent the temperature of the substance
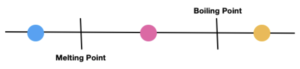
- Blue – temperature is below the melting point = substance is a solid
- Pink – temperature is between melting and boiling point = substance is a liquid
- Yellow – temperature is above boiling point = substance is a gas
Worked example:- The melting point of oxygen is -219°C and its boiling point is -183°C. Determine the state of oxygen at -200°C.
Answer: Determine if the given temperature is above the melting and/or boiling points.
-200°C is above the melting point but below the boiling point.
Therefore, oxygen is a liquid.
Solubility
Understanding Solubility
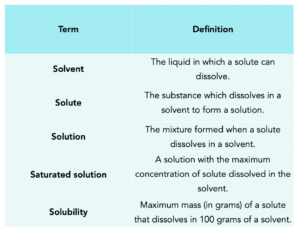
There are several definitions you need to be familiar with to understand solubility, as seen in the table below.
- The solubility of solids can be affected by temperature. As the temperature of the solvent increases, the solubility increases. For example, sugar becomes more soluble in hot water as the temperature increases.
- The solubility of gases can be affected by temperature and pressure. Unlike solids, as the temperature of the solvent increases, the solubility of gases decrease. However as the pressure increases, the solubility of gases increase.
Solubility Curves
The solubility of solids can be plotted against the temperature to produce a solubility curve.
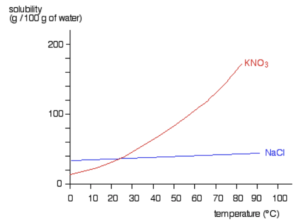
A rising curve shows that as the temperature of the solvent increases, the solubility of the solute increases.
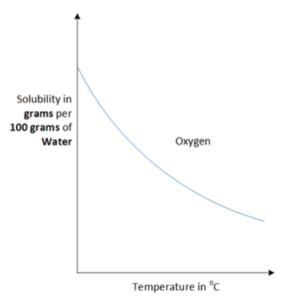
Solubility graphs for gases may look different to solubility graphs for solids. As the temperature increases, the solubility general decreases the curve produces a downwards slope.
Investigating the Solubility of a Solid at a Given Temperature
The solubility of a solid in a solvent can be investigated at a specific temperature.
Method
- Gather your equipment. You will need a beaker, boiling water, thermometer and your chosen solute and solvent.
- Heat the water. Take a known volume of water and heat it to a specific temperature. Check the temperature is correct using a thermometer and keep it in the beaker throughout the experiment.
- Gradually add the solute. Add small amounts of the solute bit by bit.
- Stop when solute dissolves. When no more solute dissolves and it remains at the bottom of the beaker, stop adding the solute.
- Record mass of solute. Record the mass of the solute that was added until the point where no more dissolved.
- Repeat. Repeat the experiment with different temperatures of the solvent and record your findings. This can then be plotted on a solubility curve.
FAQs
Changing state is the process by which matter transitions from one state (solid, liquid, or gas) to another state. This can occur through heating or cooling, and is caused by changes in the energy of the particles that make up the matter.
Melting is the process by which a solid substance transitions into a liquid state. This occurs when the substance is heated and the particles gain enough energy to break their bonds and move more freely.
Boiling is the process by which a liquid substance transitions into a gaseous state. This occurs when the substance is heated and the particles gain enough energy to break their bonds completely and become a gas.
Condensation is the process by which a gas transitions into a liquid state. This occurs when the gas is cooled and the particles lose energy, causing them to come together and form a liquid.
Freezing is the process by which a liquid substance transitions into a solid state. This occurs when the substance is cooled and the particles lose energy, causing them to form a crystalline structure and become a solid.
Sublimation is the process by which a solid substance transitions directly into a gaseous state without passing through the liquid state. This occurs when the substance is heated and the particles gain enough energy to break their bonds and become a gas.
Deposition is the process by which a gas transitions directly into a solid state without passing through the liquid state. This occurs when the gas is cooled and the particles lose energy, causing them to come together and form a solid.
Evaporation is the process by which a liquid substance transitions into a gaseous state at a temperature below its boiling point. Boiling is the process by which a liquid substance transitions into a gaseous state at its boiling point.
When a substance changes state, it can have a significant effect on its volume and density. For example, when a substance transitions from a liquid to a gas, its volume can increase dramatically. When a substance transitions from a gas to a liquid, its volume can decrease dramatically. However, the mass of the substance remains the same, so its density can also change.
Understanding changing state is important in chemistry because it is a fundamental property of matter. It can also be used to explain many physical and chemical phenomena, such as the behavior of gases, the properties of materials, and the operation of engines and other machinery. It is also important for practical applications, such as the design of refrigeration systems, the production of materials, and the synthesis of chemical compounds.





Still got a question? Leave a comment
Leave a comment