Representing Elements (GCSE Chemistry)
Representing Elements
Representing Elements in the Periodic Table
In the periodic table, each element is represented using a symbol and is surrounded by two numbers.
- The number at the top of the symbol is the mass number, which is the number of protons plus the number of neutrons within the atom.
- The number at the bottom of the symbol is the atomic number, which is the number of protons within the atom.
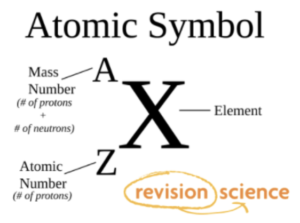
Figure X. Argon. The element argon is represented as the symbol Ar within the periodic table. The number at the top represents the mass number (40) and the number at the bottom represents the atomic number (18).
Practice Question:
- Be2+ has 7 electrons
- H+ has no electrons
- Se2- and Fe together have 77 electrons.
- The atomic number of Be2+ is different to that of Be.
- The mass number of H+ is different to that of H.
Which of the above statements are true?
- False – Be2+ has 2 electrons. As an uncharged element, it has 4 electrons. However, as it is positively charged with a +2 charge, it loses 2 electrons (4-2=2)
- True – H+ has no electrons. This is correct, as H only has 1 electron. When it’s positively charged, it loses this electron.
- False – Se2- and Fe together have 62 electrons. Se2- has 34 + 2 = 36 (as 2 electrons gained due to -2 charge). 36 + 26 (electrons in Fe) = 62.
- False – The atomic number of Be2+ is the same as that of Be, as the atomic number is the number of protons, which do not change with electron number changes. If the proton number changes, the element would differ.
- False – The mass number of H+ is the same as that of H, as there are the same number of protons and neutrons in both uncharged and charged forms of H.





Still got a question? Leave a comment
Leave a comment