Group 1: Reactivity (GCSE Chemistry)
Group 1: Reactivity
Group 1 – trend in reactivity
Trend in reactivity in a group can be explained using the electronic structure of atoms. Looking at the reactions discussed beforehand, when going down group 1 the reactions get more and more vigorous. This suggests that as you go down group 1, the reactivity of the elements increases. So lithium would be the least reactive and francium would be the most reactive out of all the elements in group 1.
Explaining the trend in reactivity
As you go down the group the reactivity of the Group 1 increases because:
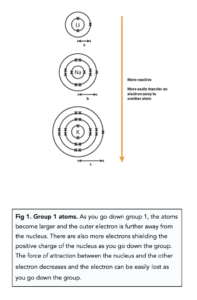
- The atoms become larger. This is due to the number of shells increasing. Therefore, the outer electron is further away from the positive nucleus, as you go down the group. The electrostatic attraction of the outer electron to the positive nucleus decreases as you go down group 1.
- The number of electron shells increases. This increases the shielding effect the positively charged nucleus has on the outer electron. Therefore, the attraction of the outer electron to the nucleus decreases as you go down group 1.
Therefore, as you go down the group the outermost electron becomes easier to lose and the reactivity increases as you go down the group.





Still got a question? Leave a comment
Leave a comment