Calculating Percentage Yield (GCSE Chemistry)
Calculating Percentage Yield
Calculating Percentage Yield
We can use a simple equation to help us calculate percentage yield.

Practice Question: This is a two part percentage yield question.
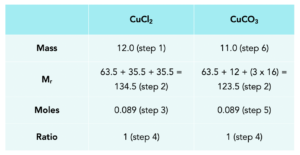
a) Jane wanted to make 12.0g of copper chloride, using the following reaction.
CuCO3(s) + 2HCl(aq) -> CuCl2(aq) + H2O(l) + CO2(g)
Calculate the mass of copper carbonate Jane should use to react with dilute hydrochloric acid to form 12.0g of copper chloride.
- Add the theoretical yield of CuCl2 to the table.
- Calculate the Mr of both compounds.
- Calculate the moles for CuCl2.
- Insert the ratios from the balanced equation.
- Insert the moles of CuCO3.
- Insert the ratios from the balanced equation.
Mass = moles x Mr
Mass of CuCO3 = 11.0g.
b) The percentage yield of copper chloride in this reaction is 81.3%. Calculate the actual mass of copper chloride made by Jane.
1.Identify the correct equation.
Percentage Yield = (Actual mass of product / Maximum theoretical product mass ) x 100
2. Rearrange the equation.
Actual mass of product = % yield x Maximum theoretical mass of product /100
3. Substitute in the numbers.
Actual mass = (81.3 x 12)/100 = 9.756g
Actual mass of copper chloride produced = 9.76g (3sf)
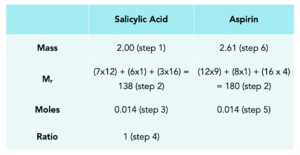
Practice Question: Susan is making aspirin using salicylic acid. She wants to calculate the maximum mass of aspirin which can be made from 3.5g of salicylic acid.
C7H6O3 + C4H6O3 -> C9H8O4 + CH3COOH
Salicylic acid Aspirin
For this question we can use the table to work out the mass of aspirin which might be formed.
- Add the mass of salicylic acid.
- Calculate the Mr of both compounds.
- Calculate moles for salicylic acid.
- Insert the ratios from the balanced equation.
- Insert the moles of Aspirin.
- Work out the mass of aspirin using Mass = moles x Mr.
Aspirin made = 2.61g
Factors Affecting Yield
Various factors can affect the yield of a reaction, meaning that we don’t always get 100% yield of our products.
- Unexpected products can form. Sometimes, reactants can produce products that we don’t expect, or even ‘unwanted’ products.
- Reactions can be reversible. Reversible reactions are said to be in equilibrium. This means that they shift between the forwards and backwards reaction, leading to products re-forming the reactants during equilibrium
- Reactants can be lost during the reaction. Reactants and products can be lost during handling or moving of products in an experiment. It’s common for some product to be left in the reaction vessel by accident after the reaction finishes
- Reactants may be impure. Some reactants might not be pure, meaning other substances are present which can react and form undesired products. When separation of the reaction mixture occurs, some of the desired product may be lost.
Percentage Yield is a term used in Chemistry to describe the efficiency of a chemical reaction. It is calculated by dividing the actual yield of a reaction by the theoretical yield and multiplying by 100 to express the result as a percentage.
Percentage Yield is calculated using the following formula: Percentage Yield = (Actual Yield ÷ Theoretical Yield) x 100. The actual yield refers to the amount of product that is actually produced in a reaction, while the theoretical yield refers to the maximum amount of product that could be produced based on the amount of reactants used.
Percentage Yield is important because it allows chemists to determine the efficiency of a chemical reaction. A high Percentage Yield indicates that the reaction is highly efficient and that most of the reactants have been converted into product. On the other hand, a low Percentage Yield indicates that the reaction is inefficient and that some of the reactants may have been lost or degraded during the reaction.
There are several factors that can affect Percentage Yield, including reaction conditions, impurities in the reactants, and loss of product during isolation and purification. In addition, the stoichiometry of a reaction can also impact Percentage Yield, as the theoretical yield is based on the balanced chemical equation.
Percentage Yield can be improved by carefully controlling reaction conditions and removing impurities from the reactants. In addition, optimizing the isolation and purification of the product can also increase Percentage Yield by reducing loss of product during these steps.
A theoretical yield refers to the maximum amount of product that could be produced in a chemical reaction based on the amount of reactants used. It is calculated using the balanced chemical equation and the stoichiometry of the reaction.
An actual yield refers to the amount of product that is actually produced in a chemical reaction. This amount can be determined by weighing or measuring the product after the reaction has been completed.
Stoichiometry is related to Percentage Yield because it determines the theoretical yield of a reaction. The balanced chemical equation and the stoichiometry of a reaction provide information about the ratio of reactants to product, which is used to calculate the theoretical yield. The actual yield of a reaction is then compared to the theoretical yield to calculate the Percentage Yield.






Still got a question? Leave a comment
Leave a comment