Alcohols (GCSE Chemistry)
Alcohols
Alcohols
Functional Groups
- The ‘O-H’ bond is the functional group of alcohols. Alcohols contain a bond between O-H, where the oxygen is bonded to a carbon. This is their functional group, used in reactions.
- As we have seen with alkanes and alkenes, alcohols can also be named. The first four alcohols in the homologous series are called methanol, ethanol, propanol and butanol.
Representing Alcohols
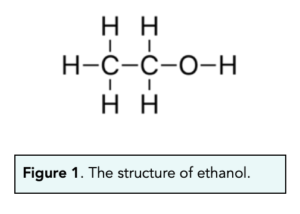
- Alcohols can be represented. We can represent alkenes very easily, by using the number of carbons each molecule contains. Ethanol can be represented as CH3CH2OH.
- Alcohols can be drawn out. We can also represent alcohols using their displayed formulae. For example, the displayed formulae of ethanol is shown below.





Still got a question? Leave a comment
Leave a comment