Isotopes (GCSE Chemistry)
Isotopes
Isotopes
- What are isotopes? Isotopes are atoms of the same element with the same number of protons but different number of neutrons in the nucleus. So isotopes have the same atomic number but different mass numbers.
- Physical properties of isotopes are different. Isotopes can have varying physical properties, because mass determines physical properties such as density, boiling and melting point.
- Chemical properties of isotopes are pretty similar. Chemical properties are determined by the number of electrons, which is the same in isotopes. Therefore they have the same chemical properties.
- Isotopes can be radioactive or non-radioactive. Radioactive isotopes have many uses. Common uses include use in medical imaging or in carbon dating.
Example: Isotopes of Chlorine
Chlorine-35 and Chlorine-37 have the same number of protons (and electrons), but have a different number of neutrons. Therefore the mass number is different. They are both the same element, so have the same atomic number.
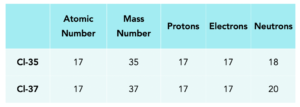
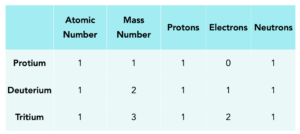
Example: Isotopes of Hydrogen
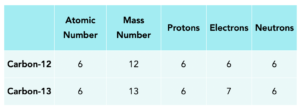
Example: Isotopes of Carbon
Practice Question: Magnesium has several different isotopes, including Mg-24, Mg-25 and Mg-26. Which of the following statements about Magnesium’s isotopes are true:
- They have the same number of protons
- They have the same number of neutrons
- They have a different number of electrons
- They have a different mass
- They have the same atomic number
- They have similar chemical properties.
- Isotopes only differ in neutron number, not protons.
- Different isotopes have different numbers of neutrons
- They have the same number of electrons, as their atomic numbers are the same.
- This is as their mass number differs, due to the variation in neutron number.
- They have the same atomic number, as the number of protons is invariant.
- They have similar chemical properties, as the electron number is the same.





Still got a question? Leave a comment
Leave a comment