Relative Atomic Mass (GCSE Chemistry)
What is Relative Atomic Mass (RAM)?
The relative atomic mass (Ar) is the average mass of atoms of an element relative to the mass of an atom of carbon-12 (which is given a mass exactly of 12). The average mass must take into account the proportions of naturally occurring isotopes of the element.
Scientists decided that the atomic mass of an atom of an element would be compared to carbon-12. Therefore the atomic mass is known as relative as it is being compared to the mass of carbon-12. If the Ar of an atom is lower than 12 it has a mass smaller than carbon-12 atom.
You can find the relative atomic mass of an element on a periodic table by looking at the number directly above the element symbol. For example the relative atomic mass of Copper (Cu) is 29.
How to Calculate Relative Atomic Mass
The relative atomic mass of an element is the average mass of an atom, and it takes into account the masses of each isotope and their proportions in the environment.
To calculate the relative atomic mass you require the following information:
- The abundance of each isotope which tells you the quantity the isotope is present in the environment. This is different for each isotope of an element.
- The mass number of each isotope of a particular element. The number of protons in all the isotopes of an atom remains the same, it is only the number of neutrons.
Lets work through an example:
Practice Question: A sample of chlorine gas is a mixture of 2 isotopes, chlorine-35 and chlorine-37. These isotopes occur in specific proportions in the sample i.e. 75% chlorine-35 and 25% chlorine-37. Calculate the relative atomic mass of chlorine in the sample.
1. Identify the isotopes. Both isotopes present in the mixture or environment, and the abundance of each isotope of the element.

2. Identify the mass number. For each isotope of the element.
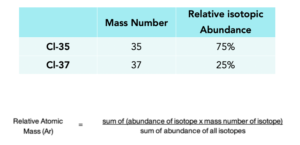
3. Write out the relative atomic mass formula to calculate relative atomic mass.
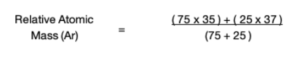
4. Substitute identified data. Substitute into the formula and work out the answer.
= 35.5
The relative atomic mass of chlorine in this mixture was 35.5. This number is closer to 35 compared to 37 as chlorine-35 is more abundant than chlorine-37.
We can do a sense-check, to make sure that the value seems right. 35.5 is in between 35 and 37, as we would expect, and it has a closer mass to Cl-35, which is the more abundant isotope. This seems fine!
You need to be able to rearrange the relative atomic mass equation to work out a missing abundance or mass.
Worked example – relative atomic mass of oxygen. Oxygen has three isotopes. The abundances in percentage are given here. The mass of one of the isotopes is unknown. The average atomic mass of the isotopes is 16.65. Work out the values of x and y.
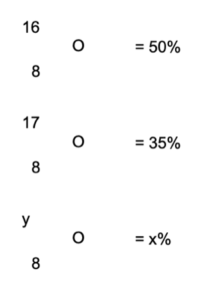
Answer:
1. Set up a table. Put in all the values as shown.
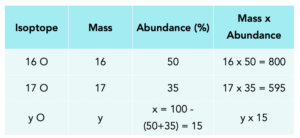
2. Work out x.
All percentages = 100, so x = 100-(50+35) =15%
3. Form an equation for y. Use the given atomic mass and the equation for calculating it.
16.65 = (800 + 595 + 15y) / 100
4. Rearrange to find y.
[16.65 x 100 – (800+595)] / 15 = y = 18
So the atomic mass of the unknown isotope = 18 and the abundance = 15%
Relative atomic mass is a measure of the average mass of an atom of an element compared to a standard unit of mass. It is used to compare the masses of different atoms and elements.
Relative atomic mass is calculated by adding up the masses of all the isotopes of an element and then averaging them based on their relative abundance in nature. This gives us an estimate of the average mass of an atom of that element.
Relative atomic mass is important in chemistry because it helps us understand the properties of different elements and how they behave in chemical reactions. It also helps us predict the masses of molecules and compounds made from different elements.
Atomic mass is the actual mass of an individual atom, whereas relative atomic mass is an average of the masses of all the isotopes of an element. Atomic mass is a specific value, whereas relative atomic mass is a relative value.
Relative atomic mass is used in chemical calculations to determine the number of moles of a substance, which can then be used to calculate the masses of other substances involved in a reaction. This information can be used to make predictions about the yields and outcomes of chemical reactions.
The standard unit of relative atomic mass is the atomic mass unit (amu), which is defined as 1/12 of the mass of a carbon-12 atom. This unit allows us to compare the masses of different atoms and elements on a relative scale.
The periodic table is arranged based on the relative atomic masses of the elements. This arrangement allows us to easily compare and predict the properties and behavior of different elements based on their position in the table.
Elements with lower relative atomic masses tend to be more reactive than elements with higher relative atomic masses. This is because lighter elements have more electrons available to participate in chemical reactions, making them more reactive.





Still got a question? Leave a comment
Leave a comment