Discovery of Protons & Neutrons (GCSE Chemistry)
Discovery of Protons & Neutrons
Protons and Neutrons
Discovery of Protons

At this stage, the model showed that there was a centre of positive charge, with electrons orbiting in different levels. However, the centre of positive charge was not understood fully.
Further experiments led to show that the nucleus could be subdivided into smaller particles, each having the same positive charge. These individual positively charged particles were called protons.
Discovery of Neutrons

In 1932 James Chadwick found evidence to support the existence of neutrons. He suggested that the nucleus consists of neutrons which have a mass but no charge. This was roughly 20 years after the concept of a nucleus was accepted within the scientific community.
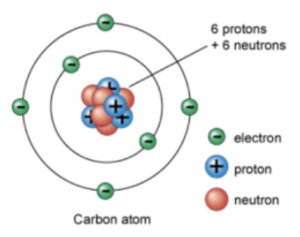
This model showed that an atom consisted of electrons orbiting around a central nucleus, consisting of protons and neutrons. Neutrons have no charge but have a mass.





Still got a question? Leave a comment
Leave a comment