Atmospheric Oxygen (GCSE Chemistry)
Atmospheric Oxygen
Oxygen Production
The Role of Plants and Algae
- Plants and algae produced oxygen. Using the process of photosynthesis, plants and algae produced oxygen. This oxygen was released into the atmosphere.
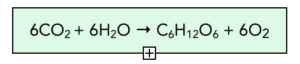
- Photosynthesis produces oxygen and glucose. In the reaction of photosynthesis, oxygen and glucose are the products. We can represent photosynthesis using a chemical reaction.
Increasing Oxygen Levels
- Oxygen was produced 2.7 million years ago. Oxygen was first produced by algae, about 2.7 million years ago. This process meant that oxygen was released into the atmosphere.
- Oxygen levels gradually increased. After oxygen was first released into the atmosphere, its levels gradually increased. This occurred due to a larger number of green plants photosynthesising.
- Increased oxygen allowed animals to evolve. Animals are complex organisms, with a large oxygen requirement. The increasing levels of oxygen in the atmosphere over the next billion years meant that animals could evolve.
Determining the Approximate Percentage by Volume of Air of Oxygen
We can determine the approximate percentage by volume of air of oxygen using metals (e.g. iron) and non-metals (e.g. phosphorus).
Using Metals
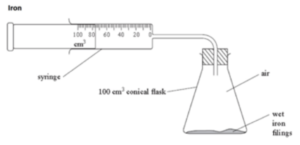
- Set up the apparatus. You will need to attach a syringe to a conical flask, as shown in the diagram below. Place wet iron fillings at the bottom of the flask.
Using Non-metals
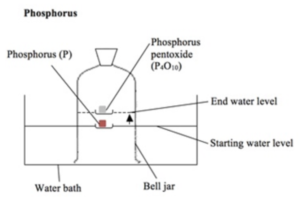
- Set up the apparatus. You will need to place an evaporating dish holding solid phosphorus onto a bath of water.
- Ignite and cover the phosphorus. Light the phosphorus with a hot wire. The floating evaporating dish can then be covered with a bell jar, as seen in the diagram below.





Still got a question? Leave a comment
Leave a comment