Volume of Gases Using Moles (GCSE Chemistry)
Volume of Gases Using Moles
Understanding the calculation of gas volumes using moles is a fundamental concept in GCSE Chemistry. This knowledge is not only crucial for excelling in exams but also provides a practical foundation for future studies and even for gaining valuable Chemistry work experience in various scientific fields.
Volumes of Gases Using Moles
- Gases take up a certain volume. If gases are at room temperature (20°C) and pressure (1 atm), then they will take up 24 dm3 of space.
- 1 mole of gas occupies 24 dm3. If we have one mole of gas at room temperature, then it has a volume of 24 dm3. This volume is also known as the molar gas volume, applying to all gases.
This means we can work out the volume of any gas at room temperature and pressure with an equation.
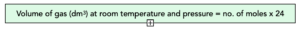
Practice Question: Calculate the volume of 0.75 mol of H2 at room temperature and pressure. (Molar volume = 24 dm3)
1.Identify the correct equation.
Volume = moles x 24
2. Substitute in the numbers.
Volume = 0.75 x 24
Volume = 18 dm3
Volumes of Gases Using Mass and Mr
We can use a different equation if we are given the mass of the gas instead of the volume.
Since moles = mass/ Mr, we can substitute moles for mass/Mr. This leaves us with the following equation.

Practice Question: Mary has calculated the mass of fluorine as 425g at room temperature and pressure. Work out the volume of fluorine at room temperature and pressure. (Molar volume 24 dm3)
1.Identify the correct equation.
Volume at rtp = (mass/Mr) x 24
2. Calculate the Mr of fluorine.
Remember, Group 7 elements are diatomic. This means that they are always in pairs
19 x 2 = 38
3. Substitute in the numbers.
Volume = (425/38) x 24
Volume of fluorine at room temperature and pressure = 268.42 dm3
Volume is a measure of the amount of space occupied by a substance. In chemistry, volume is an important property that can be used to identify and quantify substances.
In chemistry, the relationship between volume and moles is expressed by the Ideal Gas Law, which states that the volume of a gas is proportional to the number of moles of gas present, at a constant temperature and pressure.
A mole is a unit of measurement in chemistry that represents the number of particles in a substance. One mole of a substance contains Avogadro’s number of particles, which is approximately 6.022 x 10^23 particles.
We use moles to calculate the volume of gases by using the Ideal Gas Law, which states that the volume of a gas is proportional to the number of moles of gas present, at a constant temperature and pressure. By knowing the number of moles of a gas and the temperature and pressure conditions, we can calculate the volume of the gas.
The Ideal Gas Law equation is: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
It is important to use moles to calculate the volume of gases because the volume of a gas is directly proportional to the number of moles of gas present, at a constant temperature and pressure. By expressing the volume of a gas in terms of moles, we can compare the volumes of different gases in a meaningful way, regardless of the actual number of particles present.
The gas constant, R, is a constant of proportionality in the Ideal Gas Law equation. It has a value of 8.31 J/mol*K. The gas constant is used to convert between volume, pressure, temperature, and moles in the Ideal Gas Law equation.
It is important to understand volume of gases using moles because it is a fundamental concept in chemistry and is used in a wide range of applications, including the study of atmospheric chemistry, combustion reactions, and chemical engineering. By understanding how to calculate the volume of gases using moles, students will have a solid foundation for further studies in chemistry and related fields.






Still got a question? Leave a comment
Leave a comment