Reaction Profiles & Activation Energy (GCSE Chemistry)
Reaction Profiles & Activation Energy
Activation Energy
- Reactions require particle collisions. Reactions occur when particles collide with each other. The colliding particles must have sufficient energy in order to collide with each other to break the reactant bonds and cause a reaction.
- Activation energy marks a threshold. Activation energy is the minimum amount of energy that particles must have in order to react. In this way, activation energy marks a ‘threshold’. Particles that do not have the activation energy will not be able to react. The collisions are not successful.
Reaction Profiles
- Reaction profiles show energies. When we draw a reaction profile of a particular reaction, we are able to see the energy of the reactants, energy of the products and the activation energy. This will allow us to work out the overall energy change of a reaction.
Drawing Reaction Profiles
- Reaction profiles show relative energies. When drawing a reaction profile, we should be able to label the relative energies of the reactants as compared to the products.
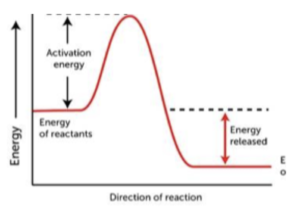
- In exothermic reactions, reactants have a higher energy than products. When we draw a reaction profile diagram for an exothermic reaction, we find that the reactants are at a higher energy level compared to the products. This is because energy is released to the surroundings during the reaction.
- In endothermic reactions, products have higher energy than reactants. When we draw a reaction profile diagram for an endothermic reaction, we find that the products are at a higher energy level compared to the reactants. This is because energy is taken in from the surroundings during the reaction.
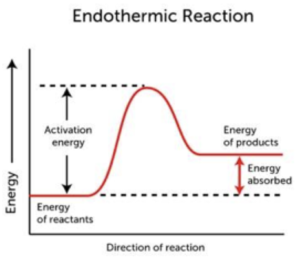
- A curved line joins reactants to products. When drawing a reaction profile, once we have labelled the energies of the reactants and products, we can join the two together with a curved line. The top of the curve indicates the activation energy needed for a reaction to occur, as shown in the profiles above.
- The curve represents activation energy. The higher the curve, the higher the activation energy needed for a reaction to occur. We can reduce the activation energy needed for a reaction to occur by using a catalyst.
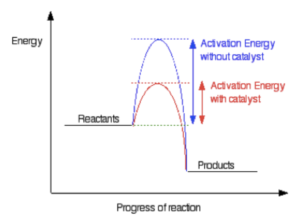
A reaction profile is a graph that shows the changes in energy that occur during a chemical reaction. It helps to visualise the progress of the reaction and the activation energy required for the reaction to take place.
Activation energy is the minimum amount of energy required for a chemical reaction to occur. It is the energy needed to overcome the energy barrier between the reactants and the transition state, which is the point where the reactants change into the products.
The rate of a chemical reaction depends on the activation energy. A reaction with a low activation energy will occur quickly and easily, while a reaction with a high activation energy will occur more slowly.
The temperature of a reaction affects the rate of the reaction by influencing the activation energy. As the temperature increases, the activation energy required for the reaction to occur decreases, leading to an increase in the rate of the reaction.
The activation energy of a chemical reaction can be determined through experiments, such as the Arrhenius equation, which uses the rate constant and temperature to calculate the activation energy.
An exothermic reaction is a chemical reaction that releases energy in the form of heat. This means that the energy of the products is lower than the energy of the reactants.
An endothermic reaction is a chemical reaction that requires energy in the form of heat. This means that the energy of the products is higher than the energy of the reactants.
These questions and answers can help students understand reaction profiles and activation energy in GCSE chemistry and prepare them for their exams.





Still got a question? Leave a comment
Leave a comment