Atomic Models Part 2 (GCSE Chemistry)
Atomic Models Part 2
Models for Atoms
Nuclear Model (Rutherford)

Ten years later, Rutherford proposed the nuclear model based on evidence from the alpha particle scattering experiment.
Rutherford and his partners (Marsden and Geiger) fired high speed alpha particles, which are densely charged, tiny, positive particles, at a piece of very thin gold foil.
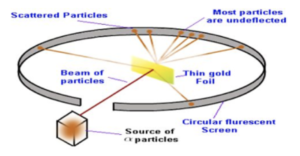
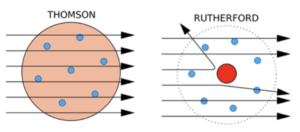
According to the plum pudding model, they were expecting all of the alpha particles to pass through undeflected because the positive charge of the atom was spread throughout the atom.
However, what actually happened was that most alpha particles went straight through the foil, whilst some alpha particles were greatly deflected and scattered.
The observations suggested the following conclusions:
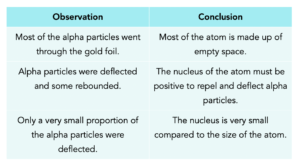
To explain these findings Ernest Rutherford proposed the nuclear model, in which there are electrons that orbit the central positively charged nucleus.
In the nuclear model here is a tiny positive nucleus, surrounded by empty space. The electrons are a long way from the nucleus in an outer orbit. Most of the mass of the atom is in the central nucleus.
If Thomson’s plum pudding model was correct, then the alpha particles should have just passed through. But in fact most alpha particles passed through, but some were deflected greatly by the positive charge in the centre of the atom. There is just empty space between the nucleus and the electrons.
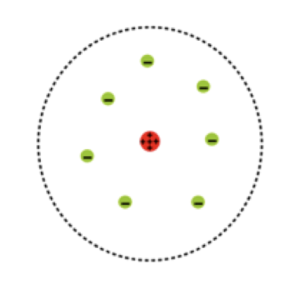
Positive charge is found in the centre (red) and electrons are found around (green).
Developed Nuclear Model (Bohr)

Neils Bohr adapted Rutherford’s nuclear model by suggesting the electrons are at specific distances from the nucleus organised in energy levels.
Bohr’s theoretical calculations supported the experimental observations. He suggested there are two electrons in the first shell, up to eight electrons in the second shell, and up to eight electrons in the third shell.
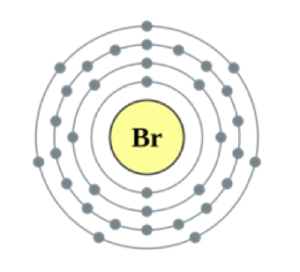
Bohr suggested that electrons orbit at specific distances (shells) from the nucleus.





Still got a question? Leave a comment
Leave a comment