Alkanes - Obtaining Alkanes (A-Level Chemistry)
Obtaining Alkanes
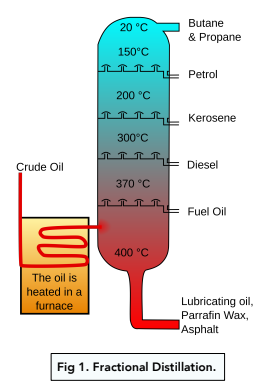
Fractional Distillation of Crude Oil
Key Terms
Petroleum is a finite fossil fuel (mostly made up of alkanes) also known as crude oil, obtained by drilling and made from dead plankton over millions of years.
Fractional distillation is the process by which mixtures of hydrocarbons in crude oil can be separated, based upon their different boiling points in a fractionating column.
Steps of Fractional Distillation
- Heat petroleum to a gas. Heat the petroleum to around 400oC at which point it becomes a gas.
- Put the gas in a fractionating column. This gas is put in the fractionating column which has a temperature gradient – it is hotter at the bottom than at the top.
- The gases condense in the column. The gases rise up the column, cool and condense at their boiling points. The liquids are prevented from falling down the column by bubble caps. The smaller the molecules are, the lower their boiling point. There are fewer Van der Waals forces between the molecules, so less energy is needed to separate them. The smaller molecules condense near the top of the column, where it is cooler.
- Remove the separated fractions. The liquids are removed from the column at different levels as mixtures of similar sized molecules known as fractions. The smallest fractions are taken off at the top of the column as gases. Each fraction has a different purpose as shown in the diagram. Most of the shorter chains are used as fuels. The longer chains are used as oils.
Modification of Alkanes by Cracking
What Is Cracking?
Cracking is the process by which larger alkanes are broken down into short-chain alkanes and alkenes. The strong covalent C-C bonds are broken during the process of cracking.
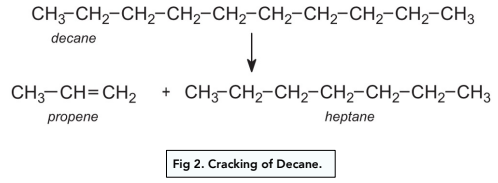
- Crude oil contains a greater amount of longer chain alkanes compared to shorter chains. Short chain alkanes are more useful as they are easier to burn as fuels and release energy, like for example petrol.
- Cracking solves the mismatch between supply and demand for short chain alkanes. A greater amount of short chain alkanes is required than is found in crude oil. Cracking produces smaller chained, more useful alkanes from less useful longer-chain alkanes.
Thermal Cracking
Thermal Cracking
- Uses very high temperatures. Between 500 and 950oC.
- Uses high pressure. Up to 7000 kPa for a very short residence time.
- Produces mainly alkenes. Thermal decomposition of the hydrocarbon chain occurs. The small alkenes are fractionally distilled to separate them. They can then be used to produce a variety of products such as polymers. Ethanol is made from any ethene produced.
Catalytic Cracking
Catalytic cracking:
- Uses a high temperature. Around 450oC. This is lower than for thermal cracking so this method is cheaper.
- Uses a low pressure. This lowers economic costs of the procedure.
- Uses a zeolite catalyst. Also known as hydrated aluminosilicate. The faster rate of the reaction contributes to making this method cheaper.
- Produces a variety of hydrocarbons. Produces more branched-chain and shorter chained alkanes, such as petrol and other fuels, and aromatic hydrocarbons (which contain a benzene ring).
Alkanes are a type of hydrocarbon, which is an organic molecule made up of only hydrogen and carbon atoms. Alkanes are characterized by having single bonds between all of their carbon atoms, and they are known for their high stability and low reactivity. In A-level Chemistry, students learn about the properties and structures of alkanes, including their names, formulas, and boiling points.
In A-level Chemistry, students learn that alkanes are important due to their widespread use as fuels, solvents, and starting materials for the production of other organic compounds. They are also important in the production of chemicals and materials used in everyday life, such as plastics, rubber, and synthetic fibers.
In A-level Chemistry, students learn about the various methods used to obtain alkanes, including extraction from petroleum and natural gas, as well as synthesis from other organic compounds. One of the most common methods of synthesizing alkanes is by the reaction of alcohols with an alkaline catalyst, such as sodium hydroxide or potassium hydroxide.
Studying the obtaining of alkanes is an important concept in A-level Chemistry as it provides insight into the production and supply of these important organic compounds. By studying this topic, students gain a deeper understanding of the methods used to obtain alkanes and the factors that influence their production and supply. This knowledge is useful in fields such as chemistry, chemical engineering, and petrochemicals, where understanding the sources and production of organic compounds is critical.





Still got a question? Leave a comment
Leave a comment