Transport Across Membranes: Osmosis (A-level Biology)
Transport Across Membranes: Osmosis (A-Level Biology)
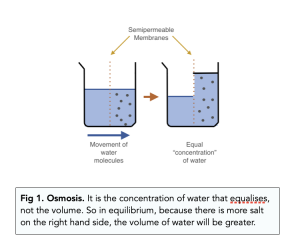
Osmosis
Osmosis is the net movement of water molecules through a semi-permeable membrane, from a region of high water potential to a region of low water potential.
-
- Osmosis is just about water. Osmosis is a term that is only used to describe the diffusion of water, and nothing else. Because water is a polar molecule, it diffuses across membranes via facilitated diffusion.
-
- Aquaporins are the water transporters. The diffusion of water across biological membranes is facilitated by special channel proteins called aquaporins. Aquaporins are designed to have hydrophilic pores which allow water molecules to move through them.
- Aquaporins are the water transporters. The diffusion of water across biological membranes is facilitated by special channel proteins called aquaporins. Aquaporins are designed to have hydrophilic pores which allow water molecules to move through them.
Water Potential
- Water moves down an osmotic gradient. Osmosis is driven by concentration gradients of water, as well as water potential. Water potential is pressure exerted by water molecules on a membrane, and this pressure is measured in kilopascals (kPa). The greater the concentration of water in a membrane, the more kinetic energy the system has, and therefore the greater the water potential.
- The units of water potential is ψw. We use the Greek letter psi, ψ, as a symbol for water potential of a solution, ψw. It has two components:
-
- Ψs is the solute potential and is a measure of the extent to which solute concentrations lower the water potential.
-
- Ψp is the pressure potential and is a measure of the pressure that develops inside of a cell as a consequence of the inflow of water.
Pure Water
- The water potential of pure water is 0. The water potential of pure water (ψw) at atmospheric pressure is zero.
- Adding solutes to water reduces the water potential. Adding solute, such as salts or glucose, reduces the concentration of the water per unit of solution. Therefore, when solutes are added to pure water, the ψw has a negative value. The more solute added to water, the more negative the water potential of pure water becomes.
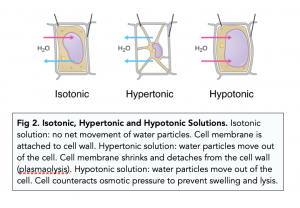
Isotonic Solution
- Osmosis is guided by water potential. In osmosis, water moves down a water potential gradient across semi-permeable membranes. Water moves from a region of high water concentration (less negative ψw) to regions of low water concentration (more negative ψw).
- Isotonic solutions have the same water potential. After osmosis occurs, the water potential of two sides of the semi-permeable membrane will be the same. Both sides are said to be isotonic.
- Hypertonic and hypotonic solutions have different water potentials. See the diagram below for further details.
FAQs
Osmosis is the passive movement of water molecules from an area of high concentration to an area of low concentration across a semi-permeable membrane.
A semi-permeable membrane is a type of biological membrane that allows the passage of small solvent molecules, such as water, while restricting the movement of larger solute molecules.
Transport across the membrane by osmosis is the movement of water molecules across a semi-permeable membrane from an area of higher water concentration to an area of lower water concentration. This process occurs spontaneously and does not require the input of energy.
In biological systems, osmosis is a critical process that helps to maintain the proper balance of fluids and electrolytes in the body. Cells are surrounded by a semi-permeable membrane that allows some molecules, such as water, to pass through while blocking others, such as ions and large molecules.
If there is a difference in the concentration of solutes (dissolved particles) on either side of the membrane, water will move across the membrane from the side with a lower solute concentration to the side with a higher solute concentration in an attempt to balance the concentration of solutes on both sides of the membrane. This movement of water continues until the concentration of solutes is equal on both sides of the membrane or until the pressure of the water on one side of the membrane becomes so high that it prevents further movement.
Osmosis can affect plant cells by causing them to become turgid or flaccid. If a plant cell is surrounded by a solution with a higher concentration of solutes than the cytoplasm of the cell, water will move out of the cell and it will become flaccid. If the concentration of solutes is lower outside the cell, water will move into the cell and it will become turgid.
The concentration gradient across the membrane affects the rate of osmosis. The greater the difference in solute concentration between the two sides of the membrane, the greater the rate of osmosis.
Yes, osmosis can occur in both animal and plant cells, as all cells have a semi-permeable membrane that allows for the passive movement of water.
In the context of osmosis, an isotonic solution is a solution that has the same concentration of solutes as the solution on the other side of a semi-permeable membrane. When two solutions are isotonic, there is no net movement of water molecules across the membrane.
In an isotonic solution, the concentration of solutes is balanced on both sides of the membrane, so there is no concentration gradient to drive the movement of water molecules by osmosis. As a result, the water molecules will diffuse across the membrane in both directions, but the net movement of water will be zero.
Isotonic solutions are important in maintaining cell shape and function in biological systems. When a cell is placed in an isotonic solution, there is no net movement of water into or out of the cell, so the cell remains in its normal shape and size. This is because the concentration of solutes inside the cell is equal to the concentration of solutes outside the cell.
No, pure water is not an example of reverse osmosis. Reverse osmosis is a process that uses pressure to force water molecules through a semi-permeable membrane from an area of high solute concentration to an area of low solute concentration, which is the opposite direction of normal osmosis.
In reverse osmosis, the membrane acts as a barrier that allows water molecules to pass through, but blocks the passage of larger molecules and ions. This results in the separation of pure water from the solutes, which are left behind on the other side of the membrane.
Reverse osmosis is used in various industrial and commercial applications, such as desalination of seawater, purification of wastewater, and production of high-purity water for pharmaceutical and electronic industries. However, the process requires energy to create the pressure that drives the water through the membrane, and it may not be as efficient in removing all types of contaminants from water.
Pure water, on the other hand, is simply water that does not contain any dissolved substances, such as minerals or ions. It can be produced by various methods, such as distillation or filtration, and does not involve the use of a semi-permeable membrane or pressure to separate the water from solutes.
Osmosis is the movement of water molecules across a selectively permeable membrane from an area of high water concentration to an area of lower water concentration, in order to equalize the concentration of solutes on either side of the membrane. Osmosis works based on the principles of diffusion and the properties of the membrane.
A selectively permeable membrane allows some molecules, such as water, to pass through while preventing others, such as ions and larger molecules, from crossing. The movement of water molecules across the membrane occurs in response to a concentration gradient of solutes, which is the difference in the concentration of solutes on either side of the membrane.
The direction and rate of osmosis are influenced by several factors, including the concentration gradient of solutes, the temperature, and the pressure. Osmosis can lead to the swelling or shrinking of cells, depending on the direction of the water movement, and can have important physiological implications for organisms.
Overall, osmosis is a fundamental biological process that plays a crucial role in regulating water balance in cells and tissues, and in maintaining homeostasis in the body.
No, osmosis is the movement of water molecules across a semi-permeable membrane from an area of higher water concentration to an area of lower water concentration. Therefore, osmosis cannot occur without water.
In osmosis, water moves from an area of high water concentration to an area of low water concentration across a semi-permeable membrane, in response to the concentration gradient of solutes (dissolved particles) on either side of the membrane. This movement of water is driven by the tendency of water molecules to move from an area of high concentration to an area of low concentration.
While other molecules can move across a semi-permeable membrane by diffusion or active transport, osmosis specifically refers to the movement of water molecules. Therefore, osmosis cannot occur without water molecules present.






Still got a question? Leave a comment
Leave a comment