Structure and Function of Antibodies (A-level Biology)
Structure and Function of Antibodies
Overview of Antibodies
We will now look more closely at how antibodies work.
- Antibodies are glycoproteins. Antibodies are designed to recognise a specific antigen. Like all proteins, the structure of an antibody determines its function and specificity.
- Antibodies are complementary to antigens. Antibodies have a specific shape which is complementary to a specific antigen.
Antibody Structure
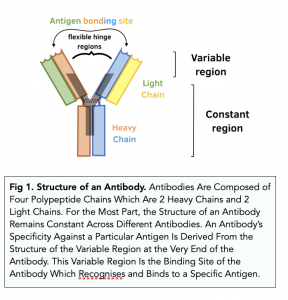
- Antibodies are Y-shaped. Each antibody is Y-shaped molecule with four polypeptide chains making it up. There are 2 long heavy chains and 2 short light chains.
- Antibodies have a constant region. Every antibody has the same constant region. The antibody uses this to bind to phagocytes.
- Antibodies have a variable region. The variable region has a unique structure that is different for each and every antibody molecule. This variable region is the antigen binding site of the antibody by which the antibody molecule can recognise and bind to a particular antigen. It is like the active site of an enzyme.
- Antibodies have hinge regions. Antibodies have flexible hinge regions, which make allow the branches of the Y to move away from each other. This makes antibodies more flexible, so can bind to multiple antigens.
Antibody Functions
Neutralisation
Antibodies can neutralise pathogens in two ways:
- Neutralising toxins. Many pathogens can produce endotoxins, which harm the host organism. Antibodies can bind to endotoxins and neutralise them.
- Neutralising antigens. Additionally, antibodies can directly neutralise viruses. Most viruses have attachment proteins that are necessary for binding to and infecting host cells. If an antibody binds to these viral attachment proteins, the virus cannot infect cells.
Agglutination
- Antibodies will clump pathogens together. Antibodies can bind to multiple antigens and therefore clump the pathogens into one big group. This big group cannot infect cells, and also makes it easy for phagocytes to engulf multiple pathogens in one go.
Marking
- Antibodies mark pathogens. Antibodies bound to a pathogen are beacons for immunological cells, and attract phagocytes and lymphocytes to the area.
Lysis
- Enzymes can work with antibodies. Enzymes can bind to antibodies bound to pathogenic antigens. These enzymes can then catalyse enzymatic reactions which break down the bacteria which are bound the antibodies.
Monoclonal and Polyclonal Antibodies
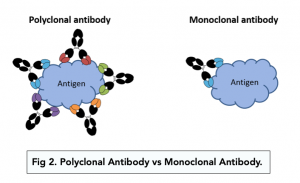
- Monoclonal antibodies have a highly specific antigen binding site and can only recognise and bind to a very unique and specific antigenic sequence.
- Polyclonal antibodies have a diverse antigen biding site. They can still only recognise a particular antigen, but they can recognise different variations of the particular antigen, such as the same antigen in different species, or mutant versions of the antigen.
Functions of Monoclonal Antibodies
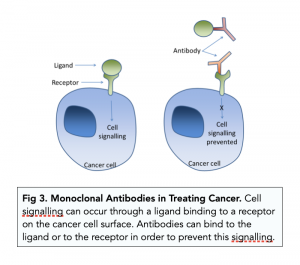
- Treating poisoning – monoclonal antibodies are used to neutralise various poisons in patients.
- Cancer treatment – cancer cells often have antigens called tumour markers. We can design monoclonal antibodies specific to these antigens, so that they can neutralise cancer cells, as well as to attract TC cells. We can also attach anti-cancer drugs to the monoclonal antibodies.
- Medical diagnosis – monoclonal antibodies can be used to detect particular antigens in patient samples of blood or tissue. For example, pregnancy testing uses monoclonal antibodies to detect for human chorionic gonadotrophin (hCG). If a woman is pregnant, hCG will be found in urine, so we can do urine tests using monoclonal antibodies.
Producing Monoclonal Antibodies
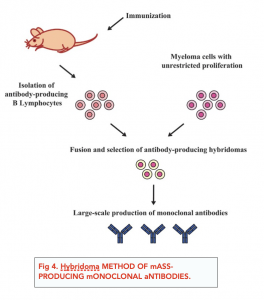
Monoclonal antibodies can be mass-produced using the hybridoma method, outlined below.
- A mouse (or another mammal) is injected with an antigen. The antigen may be a vaccine, and it may be injected several times. This causes an immune response in the mouse i.e. corresponding antibodies are produced by B cells.
- Spleen cells which are responsible for producing the lymphocytes are removed from the animal. The spleen produces B cells which are responsible for the antibody production. These spleen cells are removed from the mouse via a small operation.
- The spleen cells are fused with tumour cells to form hybridoma cells. The mouse’s spleen cells are fused with myeloma cells (cancerous white blood cells from humans), forming hybridoma cells.
- The hybridoma cells continuously produce monoclonal antibodies. These hybridoma cells have both the properties of the lymphocytes and tumour cells i.e. they are able to divide indefinitely and produce many monoclonal antibodies. These cells are checked and grown in culture in the lab.
- The monoclonal antibodies are harvested. The monoclonal antibodies produced by the hybridoma cells can be separated and harvested for further use.
Antibodies, also known as immunoglobulins, are proteins produced by the immune system in response to foreign substances, such as viruses or bacteria. Their function is to recognize and neutralize these harmful substances, called antigens, by binding to them and marking them for destruction.
An antibody is composed of four polypeptide chains, two heavy chains and two light chains, held together by disulfide bonds. The unique structure of each antibody allows it to bind specifically to a particular antigen, like a lock and key.
The structure of an antibody includes an antigen-binding site, which is composed of specific amino acid sequences that can recognize and bind to a specific antigen. Once an antibody binds to an antigen, it can neutralize it by several mechanisms, such as blocking its function, triggering its destruction, or marking it for removal by other immune cells.
The immune system is able to produce a wide variety of antibodies, each with a unique structure, by rearranging genetic material in a process called somatic hypermutation. This allows the immune system to rapidly generate new antibodies in response to new antigens, and increase the specificity and affinity of the binding between the antibody and antigen over time.
Vaccines work by introducing a harmless or weakened form of the antigen into the body, which triggers the immune system to produce antibodies against it. When the person is exposed to the real disease-causing agent, their immune system is able to recognize and quickly neutralize it, preventing or reducing the severity of the illness.
Passive immunity occurs when a person is given pre-made antibodies, either through injection or transfer of maternal antibodies through the placenta or breast milk. This provides temporary protection against antigens, but does not stimulate the production of new antibodies by the person’s immune system. Active immunity occurs when a person’s own immune system produces antibodies in response to an antigen, usually through vaccination or natural exposure to the disease. This provides long-lasting protection and memory for future responses to the same antigen.






Still got a question? Leave a comment
Leave a comment