Tests for Carbohydrates (A-level Biology)
Biochemical Tests for Sugar: Non-quantitative and Semi-quantitative
There are two types of sugars we can test for: reducing and non-reducing.
- Reducing sugars act as reducing agents in chemical reactions (i.e. donates electrons to other molecules). Reducing sugars include all monosaccharides (e.g. glucose and fructose) and some disaccharides (e.g. lactose and maltose).
- Non-Reducing sugars do not act as reducing agents in chemical reactions. They include most disaccharides (e.g. sucrose) and simple polysaccharides.
Benedict’s Test: Reducing Sugars
- Add Benedict’s solution. To test for the presence of a sugar, you add blue Benedict’s solution to your sample.
- Heat the mixture. Place the sample in a water bath and bring it to a boil.
- Observe the colour change. If your sample contains reducing sugars, the chemical reaction between the reducing sugar and the Benedict’s solution will result in the formation of a coloured precipitate (solid particles formed after a reaction between two liquids).
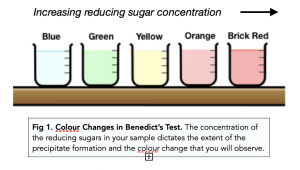
- Benedict’s Test is semi-quantitative. Benedict’s Test can give us an idea of how much reducing sugar is present in the sample. The greater the concentration of the reducing sugar, the greater the colour change in the flow diagram below. If we perform Benedict’s Test for multiple samples under standardised conditions, (i.e. ensuring all solutions were given the same amount of time from when Benedict’s solution was added to inspecting for the colour change) then we can estimate which solutions contain the greater concentration of reducing sugar in comparison to the other.
Benedict’s Test: Biochemical test for Non-reducing Sugars
No colour change (i.e. the solution remains blue), indicates a lack of reducing sugars in the sample. This could mean that there are non-reducing sugars present, or no sugars at all.
We can test further:
- Add dilute hydrochloric acid, then neutralise. The acid will break down the non-reducing sugars into monosaccharides. After adding acid, neutralise the solution with sodium hydrogencarbonate.
- Heat and observe colour change. We can then do the Benedict’s test, as we did above for reducing sugars. If there are non-reducing sugars in the sample, we will observe a colour change similar to the one we see with reducing sugars (blue to green to yellow to orange to brick red).
Reagent Test Strips: Reducing Sugars
- Reagent test strips can also detect reducing sugars. In daily practice, the presence of reducing sugars are efficiently tested for with reagent test strips.
- These test strips contain a reagent which interacts with the reducing sugar. When immersed in the test sample, the reagent in the test strip will change colour if the reducing sugar is present.
- Examples of these reagent test strips include urine test strips. These are widely used in medical settings to test for the presence of glucose in urine which occurs in Diabetes.
- The degree of colour change can indicate the concentration of the reducing sugar. The test strips are often accompanied by a colour chart which can inform the amount of reducing sugar present in the test solution. By comparing the final colour on the test strip to the colours in the chart, we can estimate the concentration of reducing sugar present in the sample.
Iodine Test: Starch
- Add aqueous iodine solution to sample. This is also known as Lugol’s solution. It is brownish-orange in colour, and is formed by adding potassium iodide to water.
- Observe the colour change. If starch is present, the solution will immediately change to a dark blue-black colour. If the colour does not change, this means that the sample did not contain and starch.
Biochemical Tests for Sugar: Quantitative
Colorimetry
As mentioned above, Benedict’s test is only a semi-quantitative technique, meaning it cannot give us an accurate concentrations of substances. To get exact values, one method we can use is colorimetry, a quantitative method that allows the concentration of compounds in a coloured solution to be determined as follows:
- A colorimeter is used to determine how much light a solution absorbs. Colorimetry involves the use of a colorimeter, a device that detects how much light is absorbed by the solution. In other words, how much of the light that is travelling through the solution is being stopped by the solution. The more concentrated the solution, the more light it will absorb.
- A calibration curve needs to first be produced. Prior to performing colorimetry on the test solution, we must first create a graph that demonstrates the degrees of light absorption at different concentrations of reducing sugars. To do this, we can measure the light absorbances of 5 glucose solutions at different known concentrations. Then plot these absorbances against the glucose concentrations and use this calibration curve to interpret the result of our test sample.
- Use the colorimeter on the test solution. Now that we have a calibration curve, we can use the colorimeter on the test sample to measure how much light is being absorbed by the solution. When you get a result, use the calibration curve to determine at what glucose concentration this degree of light would be absorbed. You now have the quantitative value of glucose concentration in your test solution.
Biosensors
Another technique which can quantify the concentration of substances in a solution is through the use of a biosensor.
- Biosensors use a biological agent to detect substances in a sample. Biosensors are devices that analyse the concentration of substances in a solution. They are called a biosensor as they contain a biological agent which interacts with the test substance. Examples of agents used in biosensors include enzymes, antibodies, nucleic acid receptors, and cellular organelles.
- The interaction between the biological agent and the test substance produces a chemical signal. If a substance which the biological agent is testing for is present in the sample solution, a reaction will occur. This leads to a chemical signal being produced, which is detected by another part of the biosensor called a transducer.
- The transducer converts the chemical signal into an electrical signal. The transducer receives this chemical signal and turns it into an electrical signal. This electrical signal can then be analysed to quantify the concentration of substance present in the sample.
- Glucose biosensors use an enzyme to measure glucose concentration in solutions. An example of a biosensor that can be used to test for sugars are glucose biosensors. They contain a glucose oxidase enzyme which oxidises the glucose in the test sample. This reaction produces a charge that is turned into an electrical signal by the transducers, which can be used to determine the value of glucose concentration in the sample.
FAQs
→Are carbohydrates sugar?
There is no difference between carbohydrates and sugar – put simply, sugar is a type of carbohydrate. There are two types of sugar; sugars that are naturally produced in foods such as fruit, and sugars that have been added in when being made e.g. cakes. Other carbohydrates include starches and fibre.
There are several tests for the above. One qualitative test for carbohydrates is Benedict’s test is used to detect sugars. To test for starch, an iodine solution can be added to the food. Foods containing starch will turn a blue/black colour.
To test for proteins, a biuret test is used.
The Sudan III test and the emulsion test are both used to test for lipids.
Simple carbohydrates (also called simple sugars) are those that include lactose, fructose and glucose. These can be found in foods such as fruit and milk.
Carbohydrates are one of the three macronutrients essential to human health and are the primary source of energy for the body. They are made up of carbon, hydrogen, and oxygen molecules and are found in a variety of foods, including sugars, starches, and fibers.
Tests for Carbohydrates are important because they allow us to identify and quantify the amount of carbohydrates present in a sample. This information is used in various fields, including food science, biochemistry, and medicine.
There are several Tests for Carbohydrates, including Benedict’s Test, Fehling’s Test, Tollens’ Test, Iodine Test, and the Seliwanoff Test. Each test uses a different reagent to detect the presence of carbohydrates in a sample.
The Benedict’s Test for Carbohydrates works by heating a sample of the carbohydrate with a solution of Benedict’s reagent. If carbohydrates are present in the sample, the reagent will turn from blue to green, yellow, orange, or brick red, indicating the presence of different types of carbohydrates.
The Fehling’s Test for Carbohydrates works by heating a sample of the carbohydrate with a solution of Fehling’s reagent. If carbohydrates are present in the sample, the reagent will turn from blue to brick red, indicating the presence of reducing sugars.
The Tollens’ Test for Carbohydrates works by adding a solution of Tollens’ reagent to a sample of the carbohydrate. If carbohydrates are present in the sample, the reagent will turn from silver to a shiny silver mirror, indicating the presence of reducing sugars.
The Iodine Test for Carbohydrates works by adding a solution of iodine to a sample of the carbohydrate. If carbohydrates are present in the sample, the iodine will react with the carbohydrate, turning the solution blue-black, indicating the presence of starch.
The Seliwanoff Test for Carbohydrates works by heating a sample of the carbohydrate with a solution of Seliwanoff’s reagent. If carbohydrates are present in the sample, the reagent will turn from pink to red, indicating the presence of ketones and aldoses.
The Tests for Carbohydrates are used in real-life applications in fields such as food science, biochemistry, and medicine. They are used to identify the presence and types of carbohydrates in food, to diagnose and monitor various medical conditions, and to study the biochemistry of carbohydrates.






Still got a question? Leave a comment
Leave a comment