Oxidative Phosphorylation and Chemiosmosis (A-level Biology)
Oxidative Phosphorylation and Chemiosmosis
Oxidative Phosphorylation
Stages of Oxidative Phosphorylation
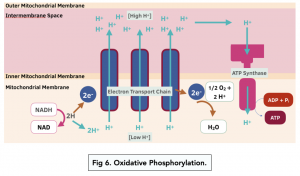
- Reduced NAD and FAD release hydrogen atoms. Reduced NAD and reduced FAD from the previous steps release hydrogen atoms in the mitochondrial matrix, and in the process they become NAD and FAD again
- Hydrogen atoms break up into protons and electrons. The hydrogen atoms split up into both H+ ions and electrons. The H+ stay in the matrix.
- The electrons enter the electron transport chain (ETC). The electrons are taken up by the electron carriers in the ETC. The electrons move along the ETC, from carrier to carrier, and at each carrier the electrons release energy.
- The energy from electrons is used to pump protons. The released energy at each carrier is used by the electron carriers to pump H+ from the mitochondrial matrix into the intermembrane space. This forms an electrochemical gradient – there is a higher H+ concentration in the inter-membrane space than in the matrix.
- Protons diffuse down the gradient. Protons diffuse from the inter-membrane space to the matrix, down the electrochemical gradient. This movement of protons is chemiosmosis.
- ATP synthase transports the protons. Protons are unable to diffuse through the phospholipid bilayer, so instead go through the enzyme ATP synthase. Proton movement provides potential energy, which rotates a section of ATP synthase, and causes phosphorylation of ADP (ADP + Pi → ATP).
- Water is formed. The electrons leave the last electron carrier and pass into the matrix, where and are accepted by oxygen. H+ also joins, forming water – a product of respiration.
Yield of ATP
For every one glucose molecule, in theory we can produce around 33 ATP molecules.
NAD and FAD
- Reduced NAD and FAD supply electrons and H+. Reduced NAD and FAD supply electrons to the ETC, and also provide H+ to help make the proton gradient for chemiosmosis.
- Each reduced NAD can make 2.6 ATP. In theory, 2.6 molecules of ATP can be made per every reduced NAD.
- Each reduced FAD can make 1.5 ATP. In theory, 1.5 molecules of ATP can be made per every reduced FAD.
Calculating the ATP Yield
For every one glucose molecule, aerobic respiration can produce:
- 10 reduced NAD. Therefore 10 x 2.6 ATP are produced = 26 ATP
- 2 reduced FAD. Therefore 2 x 1.5 ATP are produced = 3 ATP
- 4 ATP. ATP is produced directly in glycolysis and the Krebs cycle = 4 ATP.
This gives a total yield of 26 + 3 + 2 = 33 ATP
Actual ATP Yield
In reality, the maximum yield is often not obtained, for several reasons. For example, some reduced NAD and FAD can be used elsewhere in the cell for other reduction reactions, and some of the ATP can be used for transporting pyruvate into the mitochondria after glycolysis.
FAQs
Oxidative phosphorylation is the process by which cells generate ATP through the transfer of electrons from NADH and FADH2 to oxygen. This process occurs in the inner membrane of mitochondria and involves the movement of hydrogen ions across the membrane through the action of ATP synthase, a protein complex that generates ATP from ADP and inorganic phosphate.
Chemiosmosis is the process by which the movement of hydrogen ions across a biological membrane generates ATP. In oxidative phosphorylation, the flow of hydrogen ions from the intermembrane space of mitochondria back into the matrix generates a proton gradient that drives the production of ATP by ATP synthase.
The electron transport chain is a series of proteins and cofactors that transfer electrons from NADH and FADH2 to oxygen. The transfer of electrons creates a proton gradient that drives the flow of hydrogen ions across the inner membrane of mitochondria, which generates ATP through chemiosmosis. The electron transport chain is a crucial component of oxidative phosphorylation and plays a critical role in the generation of ATP.
ATP synthase is a protein complex that generates ATP from ADP and inorganic phosphate in the process of oxidative phosphorylation. ATP synthase is located in the inner membrane of mitochondria and is driven by the flow of hydrogen ions through the membrane, which generates a proton gradient that drives the production of ATP.
Oxidative phosphorylation and glycolysis are both processes by which cells generate ATP, but they differ in several key ways. Glycolysis occurs in the cytoplasm and generates only two molecules of ATP per molecule of glucose, while oxidative phosphorylation occurs in the inner membrane of mitochondria and generates many more molecules of ATP. Oxidative phosphorylation also requires the presence of oxygen, while glycolysis does not.
Oxidative phosphorylation is the final and most efficient step in cellular respiration, in which cells generate ATP from the breakdown of glucose and other fuels. This process provides cells with the energy they need to power their activities, and is crucial for the survival and growth of living organisms. Understanding oxidative phosphorylation and chemiosmosis is essential for understanding cellular respiration and energy generation in cells.






Still got a question? Leave a comment
Leave a comment