The Law of Conservation of Energy (GCSE Physics)
The Law of Conservation of Energy
Conservation of Energy
The conservation of energy is an important principle in Physics. According to this principle, we can’t ‘lose’ or ‘gain’ energy:
Energy can be transferred usefully, stored or dissipated, but cannot be created or destroyed.
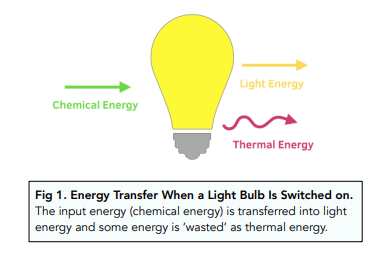
Since we know that energy cannot be created or destroyed, all the energy from a system must be dissipated somehow. The dissipated energy can be useful, or not useful (e.g. wasted energy).
In many energy transfers, thermal energy is a waste product. For example, when we switch on a light bulb, chemical energy is being transferred to light energy, but some of the energy will be ‘wasted’ as thermal energy.
Energy Transfers in a Closed System
- Closed systems don’t exchange with their surroundings. Previously, we mentioned that closed systems are unable to exchange energy to matter with their surroundings. For example, a thermos flask is a closed system as heat cannot escape (ignoring negligible amounts of heat loss).
- Energy transfers can occur in closed systems. Like any other system, energy can be transferred in a close system. However, since energy cannot exchange with the surroundings, there will be no net change to the total energy in a closed system.
- Adding ice cubes to a water bottle is an energy transfer. If you put ice cubes into a full water bottle and close the lid, you are transferring energy. We are assuming that the water bottle doesn’t allow any energy exchange with the surroundings, creating a closed system. The water will exchange thermal energy with the ice cubes, so the water will cool down.
FAQs
The law of conservation of energy states that energy cannot be created or destroyed, but it can only change form. This means that the total amount of energy in a system remains constant, even as it is transferred and transformed from one form to another.
The law of conservation of energy has a wide range of applications in the real world, including:
In mechanics, it is used to calculate the potential and kinetic energy of objects in motion.
In electrical circuits, it is used to calculate the energy transfer between components.
In thermodynamics, it is used to understand the relationships between temperature, heat, and work.
Potential energy is energy that is stored in an object due to its position or state. For example, a book on a shelf has potential energy due to its height above the ground. Kinetic energy is energy that is in motion, such as the energy of a ball rolling down a hill.
Yes, energy can be converted from one form to another, such as from potential energy to kinetic energy, or from thermal energy to mechanical energy. However, the total amount of energy in a system remains constant, according to the law of conservation of energy.
The law of conservation of energy is related to the first and second laws of thermodynamics. The first law of thermodynamics states that energy cannot be created or destroyed, only converted from one form to another, which is similar to the law of conservation of energy. The second law of thermodynamics states that the total entropy, or disorder, of a system always increases over time, which has implications for the efficiency of energy conversion processes.
The law of conservation of energy is a well-established and widely accepted scientific principle that has been supported by numerous experiments and observations. While it cannot be proven in the absolute sense, it is considered to be a fundamental principle of physics that has been thoroughly tested and supported by evidence.
The law of conservation of energy applies to renewable energy sources, such as solar, wind, and hydropower, in that these energy sources convert the energy from one form to another. For example, solar panels convert light energy from the sun into electrical energy, while wind turbines convert kinetic energy from wind into electrical energy. The total amount of energy in the system remains constant, according to the law of conservation of energy.





Still got a question? Leave a comment
Leave a comment