Isotopes (GCSE Physics)
Isotopes
Isotopes
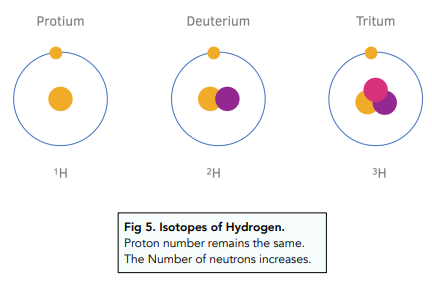
- Atoms of one element can have varying numbers of neutrons. Previously, we mentioned that atoms of the same element can have different number of neutrons. These kinds of atoms are called isotopes:
Isotopes are atoms of the same chemical element, with the same number of protons, same number of electrons but differ only in the number of neutrons they contain.
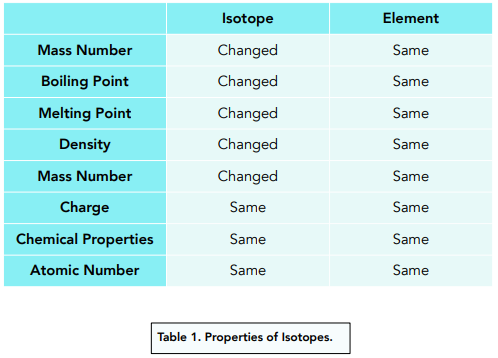
- Isotopes are different to atoms. The isotope of an element will have a different mass number to an atom of an element. This means that an isotope affects various physical properties of the element, but not the chemical properties.
Properties of Isotopes
- The physical properties of isotopes can vary. Several physical properties are determined by the mass of the atom. Such as, density, boiling point and the melting point.
- The chemical properties of isotopes are similar. They are determined by the number and arrangement of electrons which do not change.





Still got a question? Leave a comment
Leave a comment