Thermal Conduction (GCSE Physics)
Thermal Conduction
Thermal Conductivity
Conduction
Conduction is the flow of thermal energy through matters of higher temperature to matters of lower temperature.
The Transfer of Thermal Energy
- Thermal energy is transferable. Thermal energy can be transferred between objects, or from an object to its surroundings. For example, when we heat up soup in a saucepan, thermal energy will be transferred from the pan to the soup, then from the soup to the surroundings.
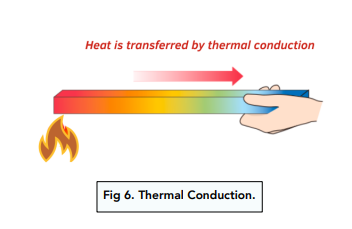
Conductors and Insulators of Heat
There are good conductors and bad conductors (insulators) of heat. We will look at some examples of both.
Good Conductors of Heat
These are helpful when heat needs to be quickly transferred. Most metals are good conductors of heat. Here are some examples:
- Silver – you can find spoons made of silver. If use a spoon to eat some hot soup, heat will be transferred to the spoon from the soup and it will become very hot.
- Aluminium – some saucepans are made of aluminium, allowing the food in the saucepan to get heated quickly.
- Iron – when we iron a shirt on an ironing board, heat from the shirt is conducted to the shirt to remove any creases.
Insulators of Heat
Insulators are often used when we want to reduce unwanted heat loss. Here are some examples of insulators:
- Air – air is a very bad conductor of heat. This explains why we find it in between the two panes of glass in double-glazed windows, as less heat will be lost.
- Wood – we often see the handles of saucepans made of wood. This means less heat will be transferred from the metal pan to the handle, so we don’t burn our hands when we hold the handle.
- Plastic – found on the handle of an electric kettle. So when you boil water to make a cup of tea, the plastic acts as an insulator so you won’t burn yourself when pouring the water into your mug.
- Wool – insulators like wool can trap air to reduce heat loss, for example in fleece winter jackets.
Investigating Thermal Conductivity
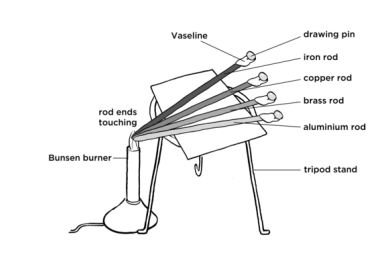
Method
- Gather the equipment. For this experiment, you will need a bunsen burner, a tripod, four rods made of different types of metal, a bench mat, a stopwatch, four matches and some paraffin wax.
- Assemble the equipment. Attach each match to a metal rod using an equal amount of paraffin wax. Place the rods over the tripod at an equal distant from each other.
- Turn on the bunsen burner. Set the bunsen burner to a low flame.
- Start the timer and heat. Bring the bunsen burner to the tip of the rods and start the timer.
- Record the time as each match drops off the rod. As the temperature of the far end reaches the melting point of the wax, the matches should drop off.
- Repeat. Repeat steps 2-5 twice more and calculate an average time for each type of metal rod. Ensure the length of the metal rods is the same.
- Record your results in a table. The results table should look like this:
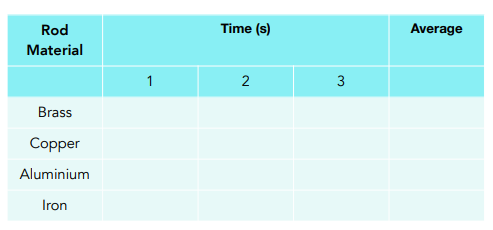
shows that thermal energy was transferred through the rod the fastest. Therefore, it is the best conductor and worst insulator.
FAQs
Thermal conduction is the transfer of heat energy through a material without any flow of the material itself.
Thermal conduction occurs due to the movement of free electrons and vibrating atoms within a material. Heat energy is transferred from the hotter end to the cooler end of the material as a result of this movement.
Examples of thermal conduction in everyday life include: the handle of a hot pan heating up, a metal spoon left in a cup of hot tea becoming hot to the touch, and a metal roof becoming hot on a sunny day.
Thicker materials generally have a lower thermal conductivity, which means they are less efficient at transferring heat energy.
Thermal conductivity is the measure of a material’s ability to transfer heat energy. It is measured in watts per metre per Kelvin (W/mK).
The factors that affect thermal conductivity include: the type of material, the temperature of the material, the density of the material, and the presence of impurities or defects in the material.
A thermal insulator is a material that has a low thermal conductivity, which means it is efficient at preventing heat energy from being transferred through it.
Examples of thermal insulators include: fiberglass, wool, Styrofoam, and air.
A thermal conductor is a material that has a high thermal conductivity, which means it is efficient at transferring heat energy through it.
Examples of thermal conductors include: metals such as copper and aluminum, and some non-metals such as graphite.
The larger the surface area of a material, the more heat energy it can transfer. This is because a larger surface area allows for more free electrons and vibrating atoms to be in contact with each other, resulting in a higher rate of heat transfer.





Still got a question? Leave a comment
Leave a comment