Movement of Particles (GCSE Physics)
Movement of Particles
Movement of Particles
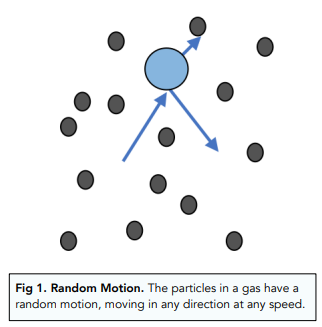
In liquids and gases, particles move randomly. This movement of particles is called the Brownian motion.
Larger sized particles may be moved by light, fast moving particles. They travel at very high speeds, so transfer a lot of energy when they collide with the smaller particles.
Movement in Gases
- Particles in a gas move constantly and rapidly. In a gas, particles are moving constantly. They move around each other randomly, at different speeds and different directions.





Still got a question? Leave a comment
Leave a comment