Pressure in Gases (GCSE Physics)
Pressure in Gases
Motion related to Pressure and Temperature
Movement related to Pressure
- Gas particles constantly collide. The particles in the gas will move around in different speeds and directions, and they will have lots of collisions. These gas particles are inside a container. The collisions will occur between two particles, or between a particle and the walls of the container.
- Colliding particles exert a pressure on the walls of a container. As the particles collide with the sides of the container, they will exert forces onto the walls of the container. All the individual forces from each collision will combine together. This combined force will exert a pressure on the walls of the container (since pressure exerted over an area will result in a force).
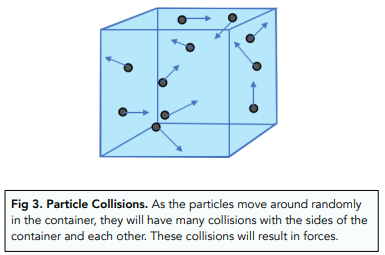
Effect of Temperature on Pressure
Increased temperature leads to an increase in pressure at a constant volume. The mechanism for this is shown in the flow chat below:
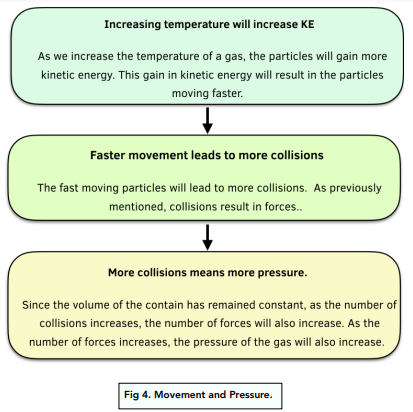
The Pressure Law
The pressure of a gas is proportional to the temperature of a gas, when measured in Kelvin (K). This is assuming the volume is kept constant.

We can express this relationship using the following equation:

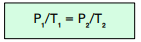
Where:
- pressure, P, in pascals, Pa
- temperature, T, in Kelvin, K
Pressure
- Pressure is affected by volume. Changing the volume of a container will affect the pressure of the gas inside it. When the volume of the container increases, the pressure of the gas will decrease.
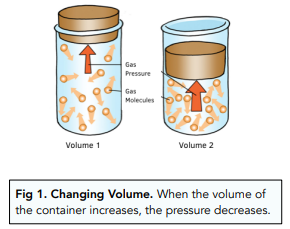
Calculating Pressure and Volume
We can calculate pressure and volume using the following equation. The constant does not change (assuming the temperature doesn’t change), which means that if the volume rises, pressure has to fall, and vice versa.

Another way of expressing this is through Boyle’s Law:
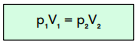
Where:
- pressure, p, in pascals, Pa
- volume, V, in metres cubed, m³
Question: The volume of a sample of gas in a sealed container is changed with the temperature remaining the same. Which statement is correct?
If the pressure is doubled, the volume also doubles.
If the pressure is halved, the volume doubles.
If the pressure is halved, the volume is also halved.
If the pressure is doubled, the volume reduces to a quarter.
Answer: Using our equation, pressure x volume = constant, we can work out that B must be the correct answer.
Work
- Work is the transfer of energy. Work done is simply another way of saying that energy is transferred. The energy is usually transferred by a force. You may wish to re-cap our previous work in 4.1 Energy.
- Doing work will increase internal energy. By doing work on a gas (for example increasing its pressure), we can increase the internal energy of the gas. Previously, we discussed that the internal energy is the combination of kinetic energy and potential energy of the particles.
- Internal energy affects temperature. As the internal energy of the particles increases, the temperature of the gas also increases. The kinetic energy has been increased and therefore the gas becomes warmer.
Example: A Bicycle Pump
Question: Yasmin is using a bike pump. As she is inflating her bike tyre, she feels the pump handle getting warmer. Why is this happening?
More particles are being forced into the tyre. As Yasmin is pumping up the bike tyre, she is making more air particles move into the tyre. She is doing work against a force for this to happen.
Kinetic energy of the particles increases. By doing work, Yasmin is transferring energy to the particles inside the tyre. This energy is converted into kinetic energy.
Increased KE leads to increased temperature. The increase in kinetic energy will result in the temperature of the tyre increasing.
Tyre is connected to the pump. The tyre is connected to the bike pump, therefore as the tyre gets warmer, Yasmin will feel the bike pump also getting warmer.
Pressure in gases refers to the force exerted by a gas on a surface per unit area. It is a measure of the amount of force exerted by the particles of a gas in a confined space.
The unit of pressure is the pascal (Pa), which is a unit of force per unit area. Other units of pressure include atmospheres (atm), kilopascals (kPa), pounds per square inch (psi), and millimeters of mercury (mm Hg).
The pressure of a gas is directly proportional to its temperature, according to the gas law known as Gay-Lussac’s law. This means that as the temperature of a gas increases, so does its pressure. Conversely, as the temperature of a gas decreases, its pressure also decreases.
The pressure of a gas is inversely proportional to its volume, according to the gas law known as Boyle’s law. This means that as the volume of a gas increases, its pressure decreases, and vice versa.
Atmospheric pressure is the pressure exerted by the weight of the Earth’s atmosphere on the surface. It is important because it affects weather patterns and the behavior of gases and liquids on the Earth’s surface. For example, changes in atmospheric pressure can cause changes in air and ocean currents, which can impact the formation of storms and weather patterns.
The pressure of gases in containers can affect their behavior in several ways. For example, if the pressure inside a container is increased, the gas particles will collide with the walls of the container more frequently, which can increase the temperature of the gas. On the other hand, if the pressure inside a container is decreased, the gas particles will collide with the walls less frequently, which can cause the temperature of the gas to decrease.
The pressure of a fluid in a container is directly proportional to its depth. This means that as the depth of a fluid increases, its pressure also increases, and vice versa. This relationship is known as Pascal’s law and is an important concept in understanding the behavior of fluids and gases in containers.
The pressure of gases in pipes and tubes can affect the flow of the gas through these systems. For example, if the pressure of a gas in a pipe is increased, the flow rate of the gas through the pipe will also increase, and vice versa. Understanding the relationship between pressure and flow rate is important for designing and optimizing gas delivery systems, such as pipelines and ventilation systems.





Still got a question? Leave a comment
Leave a comment