GCSE Specific Heat Capacity
What is the Specific Heat Capacity?
The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of one kilogram of the substance by one degree Celsius. It is very important for students to know about the specific heat capacity for GCSEs.
The specific heat capacity is different for each liquid – e.g. it takes a different amount of heat energy to raise the temperature of water by 1°C compared to a different liquid.
If a large amount of energy is required to heat up a substance, then its specific heat capacity will be very high. These substances will be able to store lots of energy.
If a small amount of energy is required to heat up a substance, then its specific heat capacity will be very low. These substances won’t be able to store lots of energy.
Specific Heat Capacity of Water = 4200 J/kg°C
Specific heat capacity of Aluminium = 900 J/kg°C
Specific heat capacity of Copper = 389 J/kg°C
Investigating Specific Heat Capacity
Specific Heat Capacity of Liquid
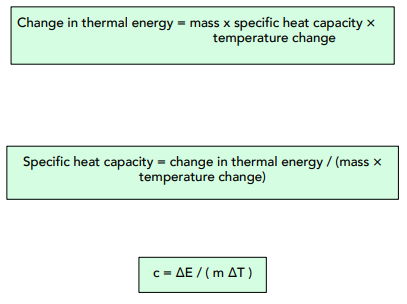
Measuring the specific heat capacity of a liquid is fairly simple. We can heat up a liquid with a known amount of thermal energy, and then observe the temperature change caused.
We can re-arrange this:
Specific Heat Capacity of Solid
We are able to calculate the specific heat capacity of a solid material too. For example, if you wanted to calculate the specific heat capacity of ice. Again, we want to heat up the solid with a known amount of thermal energy, and then observe the temperature change. You need to know a specific method for this experiment, so learn this well:
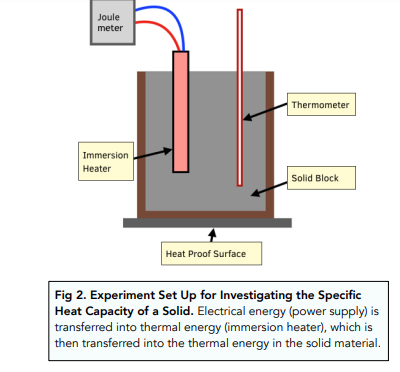
- Take a block of material. First take the block that you are going to investigate. This block is going to have two things embedded into it: the thermometer to measure the temperature of the block, and the immersion heater to provide heat energy to the block. In this experiment we are essentially seeing how much heat energy is needed (from the immersion heater) to heat up the block by a certain temperature (measured by the thermometer).
- Measure the mass of the block. You should measure the mass of the block using a top pan balance, recording the mass in kg.
- Insulate the block. Insulate the block by coating it in an insulating material, such as a sponge or cotton wool. This will help to reduce the loss of heat to the surroundings.
- Record the temperature of the block. We need to record the temperature of the block at the start of the experiment, in °C.
- Attach the immersion heater to a joule-meter. The joule-meter will measure the amount of energy supplied to the block throughout the experiment.
- Heat the block. Switch on the immersion heater and wait until the temperature of the block has risen by 10 degrees. The heater will provide thermal energy to the block, which we can measure using the joule-meter. We can measure the temperature of the block using the thermometer. Measure the final temperature of the block.
- Find the energy and temperature change. Make a note of the final temperature change (which will be around 10 degrees) and the final energy value on the joulemeter.
- Rearrange the equation. Our equation for specific heat capacity is ∆E=mc∆T. In order to find the specific heat capacity of the block, we have to rearrange to make ‘c’ the subject of the formula (like we did earlier). Therefore, c = ∆E / m∆T.
- Substitute in the numbers. Now that we have our equation, we can put in the values we have recorded. Our value of ∆E will be from the joule-meter, m is the mass of the block and ∆T is the temperature change that we measured.
- Give the specific heat capacity in J/kg°C. Once we have calculated the value for specific heat capacity, we have to give the correct units, which is J/kg°C.
Quick Fire GCSE Specific Heat Capacity Questions
- What is the specific heat capacity of water?
- What is the specific heat capacity of ice?
- What is the specific heat capacity of copper?
- What is the specific heat capacity of Aluminium?
- How to calculate specific heat capacity?
- What unit is used to measure energy when calculating specific heat capacity?
The specific heat capacity of a substance is the amount of heat energy required to raise the temperature of one kilogram of the substance by one degree Celsius. It is very important for students to know about the specific heat capacity for GCSEs.
The specific heat capacity is different for each liquid – e.g. it takes a different amount of heat energy to raise the temperature of water by 1°C compared to a different liquid.
If a large amount of energy is required to heat up a substance, then its specific heat capacity will be very high. These substances will be able to store lots of energy.
If a small amount of energy is required to heat up a substance, then its specific heat capacity will be very low. These substances won’t be able to store lots of energy.
Specific Heat Capacity is important because it helps us understand how different substances react to changes in temperature and how much energy they can absorb or release.
Some common units for Specific Heat Capacity include Joules per kilogram per degree Celsius (J/kg°C) and calories per gram per degree Celsius (cal/g°C).
Specific Heat Capacity is calculated by dividing the amount of heat energy added to a substance by the substance’s mass and the change in temperature.
Factors that can affect Specific Heat Capacity include the type of substance, its purity, and its temperature.
Yes, Specific Heat Capacity can be measured using scientific equipment such as calorimeters.
Yes, Specific Heat Capacity is used in a variety of real-world applications, such as energy management, material selection, and food preservation.





Still got a question? Leave a comment
Leave a comment