Changes in Thermal Energy (GCSE Physics)
Changes in Thermal Energy
Calculating Thermal Energy Changes in Systems
Formula for Energy Change
We can calculate the amount of energy that is stored in or released from a system using this equation:
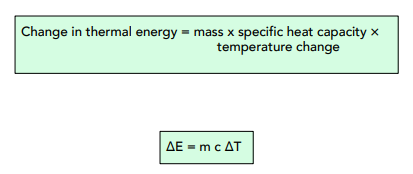
Where:
- change in thermal energy, ∆E, in joules, J
- mass, m, in kilograms, kg
- specific heat capacity, c, in joules per kilogram per degree Celsius, J/kg°C
- temperature change, ∆T, in degrees Celsius, °C
We cannot just measure the rise in temperature to find out the change in thermal energy, because the amount of heat energy needed to increase the temperature by 1°C varies dependent on the mass and type of liquid:
- The mass is accounted for in the equation
- The type of liquid is accounted for in the specific heat capacity. We will look at this below.
Question: Louisa heats up a 20kg block of concrete. She waits until the temperature has risen from 20 °C to 65 °C. Find the energy used to heat up the block, given that the specific heat capacity of concrete is 3200 J/kg°C.
- We need to write out the equation for specific heat capacity.
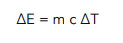
- We need to calculate the temperature change.
We have not been told this value in the question, so we need to work it out.
65 – 20 = 45 - We can use the formula.
We have all the numbers that we need, so we can simply substitute them into the formula. When we give our final answer, it may be more appropriate to give the units in terms of kilojoules.

Question: Louisa heats up a 20kg block of concrete. She waits until the temperature has risen from 20 °C to 65 °C. Find the energy used to heat up the block, given that the s.h.c of concrete is 3200 J/kg°C.
- Write out the equation for specific heat capacity.
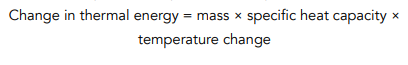
- Calculate the temperature change.

- Use the formula.
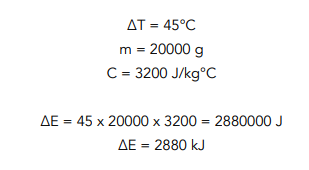
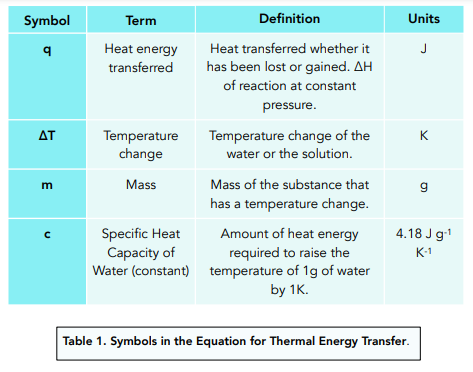
Question: A student tried to measure the enthalpy change of combustion by burning 2.2g of liquid ethanol completely in oxygen. The amount of water used in the experiment was 100g and the temperature rose from 293K to 348K. The molar mass (Mr) of ethanol is 46.1. The specific heat capacity of water is 4.18 J g-1 K-1. What is the standard enthalpy change of combustion (∆c HƟ) of ethanol?
- Write out the equation for specific heat capacity and fill in the values you know.
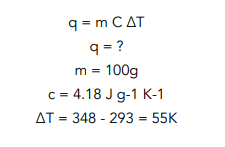
- Calculate the heat energy transferred.
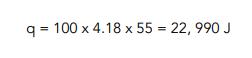
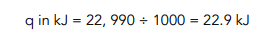
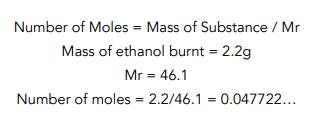






Still got a question? Leave a comment
Leave a comment