Cycles - Decomposition & The Nitrogen Cycle (GCSE Biology)
Decomposition & The Nitrogen Cycle
The Nitrogen Cycle
Decomposers play a role in the Nitrogen Cycle.
The Nitrogen Cycle is important to help recycle nitrogen from the atmosphere and it is an important element for making proteins and helping organisms grow.
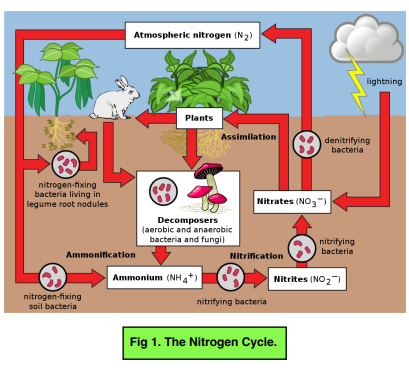
- Nitrogen Fixation – nitrogen gas from the air (78% of the air) is too unreactive to be used by plants directly. Hence, it has to be converted into a soluble form using nitrogen-fixing bacteria in the root nodules which forms nitrates. Lightening can also convert nitrogen to form nitrates.
- Nitrification – Ammonium ions in the the soil can also be converted into nitrates using nitrifying bacteria.
- Assimilation – nitrates from the soil are absorbed by the roots and are used to make amino acids and proteins which are incorporated into the plants. Animals then eat the plants and the nitrogen in the plants is transferred to proteins in the animal. Animals can then pass this to other animals through feeding.
- Ammonification – excretion from organisms (faces and urine) and death returns to the nitrogen to the soil. The nitrogen is then converted to ammonia and goes on to form ammonium ions. Nitrogen gas can also be converted into ammonia through the Haber Process so that ammonia can be used in fertilisers.
- Denitrification – the nitrates from the soil are then converted into nitrogen gas by denitrifying bacteria and this is how nitrogen is returned to the atmosphere.
Decomposition
Factors Affecting Decomposition
We began to discuss decomposition when looking at the carbon cycle. Now we must look into the topic in more detail.
Decomposition is measured by the rate of decay. The rate of decay is a measurement of the speed at which decomposers break down dead organisms.
- Temperature affects decomposition. Decomposers have an optimum temperature. If the temperature is too low, the rate of decay will reduce, as the enzymes required for decomposition will work too slowly. If the temperature is too high, the rate of decay will drop dramatically as the enzymes required for decomposition will denature and the decomposer will die.
- Water affects decomposition. Decomposers require water to survive. IF they are starved of water, the rate of decay decreases. If there is a lot of water, there will be a high rate of decay, as some decomposers use enzymes that are secreted onto dissolved organic matter and work through absorption.
- Availability of oxygen affects the rate of decay. In general, Oxygen is required to allow decomposers to respire. Decomposers must respire in order for true decomposition to take place. As more and more oxygen is made available, more and more decomposition also occurs. Some decomposers do work in anaerobic conditions.
Food Preservation and Decay
Preservation methods can decrease the rate of decay in foods.
- Airtight containers prevent the entry of microorganisms. The containers are sterilised at high temperatures and pressures to kill any present microorganisms.
- Water can be removed from foods by drying. Microorganisms are unable to survive and reproduce with the lack of water. Food can be dried by adding sugar or salt which draws out water by osmosis.
- Fridge/ freezers maintain a low temperature to slow down decay. The cool temperature reduces the rate of reproduction of decomposers.
Investigating the Rate of Decay
Decay of Milk
You can investigate the effect of temperature on the rate of decay in milk. Milk becomes acidic as it decays so you can test for the pH when conducting this experiment:
- Place 30ml of milk in 4 separate beakers.
- Measure and record their initial pH using universal indicator strips.
- Incubate each beaker at different temperatures e.g 10°C, 20°C, 30°C and 40°C.
- Test for their pH repeatedly at 24hrs, 48hrs and 72hrs and record your results. You will see that the rate of decay is faster at higher temperatures.
Calculations
We must remember that rate of change is measured as, the change in a value divided by the time period. Therefore, if we get values of decomposition, we can calculate the rate of decay.
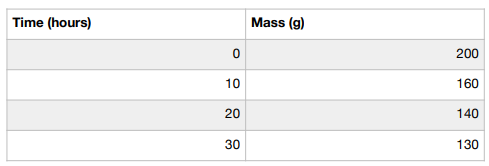
Therefore, if we wanted to calculate the rate of decomposition from start to finish using this data, we subtract 200 from 130, then divide by 30. This gives us the value of 2.33. This is the rate of change.
If we plot this data on a graph, the gradient is the rate of decay.
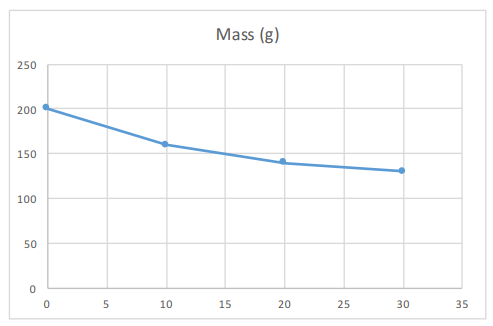
You can calculate the gradient by using the phrase, rise over run. Therefore the gradient is the change in the y axis, divided by the change in the x axis. This will also allow you to calculate the rate of decay
The Decomposition Cycle, also known as the Decay Cycle, is a natural process in which dead organic matter is broken down by decomposers such as bacteria and fungi. This process releases nutrients back into the soil, making them available for other living things to use.
The Nitrogen Cycle is the process by which nitrogen is cycled through the environment, moving from the atmosphere, to the soil, to living things, and back to the atmosphere. This cycle helps to regulate the amount of nitrogen available in the environment and is essential for the growth and survival of living things.
The Nitrogen Cycle involves four main steps: nitrogen fixation, nitrification, assimilation, and denitrification. In the nitrogen fixation step, nitrogen is converted from nitrogen gas in the atmosphere into a form that can be used by plants. In the nitrification step, the nitrogen is converted into nitrates. In the assimilation step, the nitrates are taken up by plants and incorporated into their tissues. In the denitrification step, the nitrogen is converted back into nitrogen gas and returned to the atmosphere.
The Nitrogen Cycle helps living things by making nitrogen available in a form that they can use. Nitrogen is an essential element for the growth and survival of all living things, and the Nitrogen Cycle helps to regulate the amount of nitrogen available in the environment.
The Nitrogen Cycle is important for the environment because it helps to regulate the amount of nitrogen available. Too much nitrogen in the environment can lead to problems such as eutrophication, which can cause harm to aquatic ecosystems. The Nitrogen Cycle helps to prevent these problems by cycling nitrogen through the environment in a balanced and controlled way.
Human activities such as agriculture, industry, and transportation can have a significant impact on the Nitrogen Cycle. For example, the use of fertilisers in agriculture can increase the amount of nitrogen in the soil, leading to problems such as eutrophication. Human activities can also release large amounts of nitrogen into the atmosphere, contributing to climate change.
Decomposers play a crucial role in the Nitrogen Cycle by breaking down dead organic matter and releasing nutrients back into the soil. The nitrogen in the dead organic matter is converted into a form that can be taken up by plants, which helps to regulate the amount of nitrogen available in the environment.






Still got a question? Leave a comment
Leave a comment