Atomic Structure - 4.1.3 The Development of the Model of the Atom (common content with Chemistry) (GCSE Physics AQA)
The Development of the Model of the Atom (common content with Chemistry)
Understanding atomic structure is fundamental, not only for theoretical knowledge in physics and chemistry but also for practical applications encountered in Chemistry work experience.
Discovery of the Electron
Atoms were thought to be the smallest particle before the electron was discovered. Scientists thought that it was a very small dense sphere, which could not be split into pieces.
Plum Pudding Model
- The discovery of the electron changed scientific thoughts. Once scientists discovered the electron, they changed the model of the atom. Originally, they thought that an atom was just one single sphere.
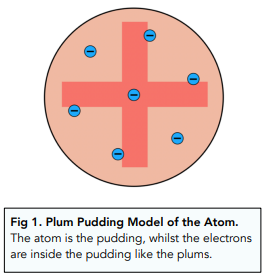
- Electrons were thought to be buried in the atom. Now that scientists knew that electrons existed, they thought that they must be buried inside the atom. They thought that the atom was positive, with negative electrons embedded inside (see figure 1).
The Nuclear Model
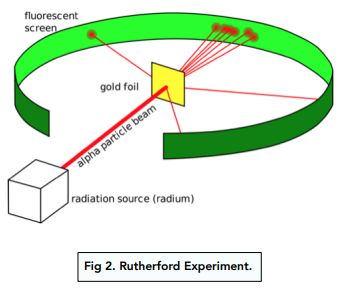
- Scientists investigated the atom further. A scientist called Rutherford investigated the atom further with his two assistants. He decided to perform an experiment, which was called the alpha particle scattering experiment.
- They aimed an alpha particle beam at a gold sheet. The scientists used a gold foil sheet, at which they fired positive alpha particles in a beam from a source. When performing the experiment, they found that some particles went through the sheet. Other particles seemed to ‘bounce off’ the sheet and come straight back to the alpha particle source, like a boomerang.
- The plum pudding model couldn’t explain the particles’ behaviour. The model of the atom at the time (the plum pudding model) couldn’t explain why some of the alpha particles came straight back to where they came from.
- They proposed a nuclear model. To explain their results, Rutherford and his team came up with a new model for the atom, called the nuclear model. This model said the following:
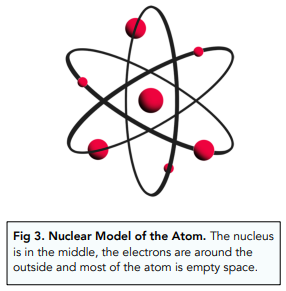
- The mass of the atom is must be concentrated at the centre of the nucleus, since only a few of the alpha particles were deflected back.
- The nucleus of the atom must be charged (positively), since it was repelling some of the positive alpha particles away.
- The nucleus of the atom must be small, since only a few of the alpha particles were repelled (not all of them).
- Most of the atom must be empty space, since most of the alpha particles passed through without being repelled by the nucleus.
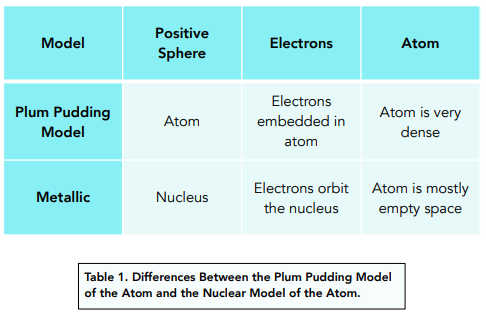
Differences between the 2 Models
Niels Bohr
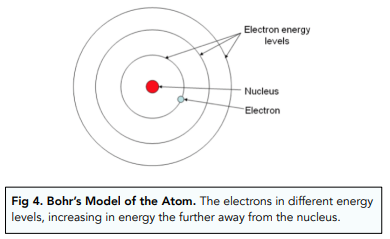
- Niels Bohr changed the nuclear model. Soon after the nuclear model was proposed, Niels Bohr adapted the model of the atom. He looked more closely at the structure of the atom, particularly the electrons.
- Bohr was looking at orbiting distances. The nuclear model has already proposed that electrons were orbiting around a small positive nucleus, but Bohr wanted to know how they were orbiting. Through various experiments, he found that each electron was orbiting the nucleus at a specific distance.
(For AQA exams, you do not need to know the specific experiments that he carried out).
- The orbiting distance was linked to energy levels. Bohr realised that the orbiting distance of the electron was linked to energy levels. Each electron had a specific energy, which meant that it orbited the nucleus at a specific distance. (Previously, we saw that the higher the energy of the electron, the further away from the nucleus it orbits).
Protons
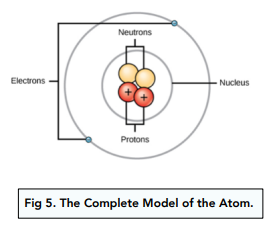
- The nucleus was made up of smaller particles. Soon after Bohr’s theory, other scientists began to look at the nucleus. Through their experiments, they found that the nucleus was actually made up of smaller particles.
- The small particles were charged. The small particles in the nucleus were found the charged. Each of the particles had a positive charge of +1. These positive particles were called protons.
- Protons determined nuclear charge. Through working out the charge of a proton, scientists knew that they could work out the charge of the nucleus. To find the overall nuclear charge, the scientists simply had to add up the charges of all the protons in the nucleus of an atom.
James Chadwick
- James Chadwick improved the nuclear model. About 20 years after the nuclear model was an accepted idea, Chadwick adapted the nuclear model again. He was also looking at the structure of the nucleus. (For AQA exams, you do not need to know the specific experiments that he carried out).
- Chadwick investigated neutrons. Chadwick was particularly interested in small particles called neutrons within the nucleus. The nuclear model at the time explained that the protons and electrons balanced out charges, but it left the atomic and mass numbers unaccounted for.
- Neutrons completed the nuclear model. Through his discovery of neutrons, James Chadwick completed the nuclear model, making it the model that we use today. Neutrons were found to be uncharged particles, but they had the same mass as protons. Now, the charges and masses were all accounted for.
Changing Scientific Models
As we have seen in this section, the nuclear model was constantly changed and adapted by different scientists. Rutherford’s particle scattering experiment was particularly revolutionary, since it brought about the first idea of a nuclear model rather than a ‘plum pudding’ model.
In the future, our current scientific models could be changed or replaced depending on new experimental evidence.





Still got a question? Leave a comment
Leave a comment