Practice Model of Water - 3.3.2 Pressure in Gases (Physics Only) (GCSE Physics AQA)
Pressure in Gases (Physics Only)
Pressure
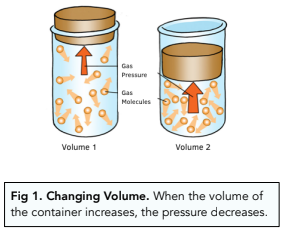
- Pressure is affected by volume. Changing the volume of a container will affect the pressure of the gas inside it. When the volume of the container increases, the pressure of the gas will decrease.
- A fall in volume leads to a rise in pressure. As the size of the container decreases, we find that there is less ‘room’ inside the container. The particles are therefore contained within a smaller volume. As a result, the particle collisions occur more frequently with the sides of the container, exerting a higher pressure.
- A rise in volume leads to a fall in pressure. The reverse of the above explanation is true when the size of the container increases. There is more space, so less frequent collisions with the walls of the container, exerting a lower pressure.
- Pressure produces a force at 90o to the walls of the container. As we learnt previously, when gas is trapped inside a container, it exerts a pressure on the walls of the container. In turn, this pressure produces forces on the walls of the container. The forces will be at right angles to the walls of the container. (This is explored in more detail in chapter 4.5, Forces).
Calculating Pressure and Volume
We can calculate pressure and volume using the following equation. The constant does not change (assuming the temperature doesn’t change), which means that if the volume rises, pressure has to fall, and vice versa.

Where:
- pressure, p, in pascals, Pa
- volume, V, in metres cubed, m³
Question: The volume of a sample of gas in a sealed container is changed with the temperature remaining the same. Which statement is correct?
If the pressure is doubled, the volume also doubles.
If the pressure is halved, the volume doubles.
If the pressure is halved, the volume is also halved.
If the pressure is doubled, the volume reduces to a quarter.
Answer: Using our equation, pressure x volume = constant, we can work out that B must be the correct answer.





Still got a question? Leave a comment
Leave a comment