Atomic Structure - 4.2.1 Radioactive Decay and Nuclear Radiation (GCSE Physics AQA)
Radioactive Decay and Nuclear Radiation
Nuclei Stability
- Nuclei have different stabilities. Some atomic nuclei are stable, whilst other atomic nuclei are unstable. The majority of isotopes have unstable nuclei.
- Decay leads to stability. In order to become stable, unstable nuclei undergo a process called radioactive decay. When a nucleus decays, it will give out radiation.
- Radioactive decay is random. The process of radioactive decay is a random process. Since it is random, we cannot predict which nuclei will decay at any given time.
Activity and Count Rate
- We can measure radioactive decay. Although it is a random process, we can measure radioactive decay. The two things we can measure are the rate of radioactive decay and the number of decays per second.
- Activity is the rate of decay. The rate of decay is called the activity of the sample. If we have an unstable sample, then we can measure the rate of decay in becquerel (Bq).
- Count rate is the number of decays. The number of decays each second is called the count rate of the sample. If we have an unstable sample, then we can measure the count rate using a detector such as a Geiger-Muller tube.

Types of Nuclear Radiation
We have previously mentioned that when a nucleus decays, it emits radiation. This radiation can take on several forms.
- Alpha particle. An alpha particle is like the nucleus of a helium atom. It is made up of 2 neutrons and 2 protons. This is emitted as an alpha particle, represented by this symbol: α.
- Beta particle. A beta particle is essentially an electron. When the nuclei is decaying, one of the neutrons in the nucleus will change into a proton and an electron. It is this electron that gets emitted as a beta particle, represented by this symbol: β.
- Gamma ray. A gamma ray is not a particle. It is actually a form of electromagnetic wave. It is this EM wave that gets emitted from the nucleus as a gamma ray, represented by this symbol: γ.
- Neutron. A neutron is a particle found in the nucleus. It can be emitted from the nucleus during radioactive decay, represented by this symbol: n.
Properties of Radiation
For AQA exams, you need to be able to remember the information from the following table:
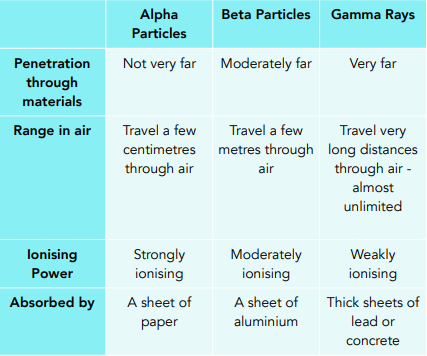

Uses of Radiation
- Radiation can be useful. We can use radiation in a variety of ways, for example to find leaks in pipes, or to treat cancer. However, we have to be careful when using radiation since it poses some risks.
- Different sources may be used. Since there are many different sources of radiation available, we need to choose carefully which source we use. Some types of radiation are more appropriate than others in certain situations.
(We will be discussing the uses of radiation in more detail later on.)





Still got a question? Leave a comment
Leave a comment