Atomic Structure - 4.2.2 Nuclear Equations (GCSE Physics AQA)
Nuclear Equations
Representing Radioactive Decay
We are able to represent radioactive decay through the use of nuclear equations. First, we need to know how to represent alpha and beta radioactive emissions:
An alpha particle is represented by:
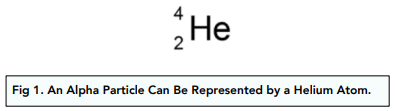
A beta particle is represented by:
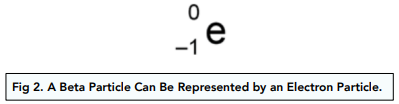
Changing the Nucleus
Through emitting radiation, we can change the nucleus of an atom. There are two ways in which the nucleus of an atom can be changed:
- Charge. We can increase or decrease the charge of an atom through nuclear radiation.
- Mass. We can decrease the mass of an atom through nuclear radiation.
Alpha Decay
Firstly, let us look at alpha decay with the following example:
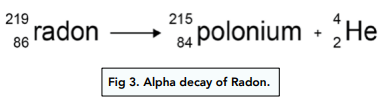
- A helium nucleus has been emitted. Since a helium nucleus has been emitted, we know that this is alpha decay. The helium nucleus is made up of 2 protons and 2 neutrons, and it is the alpha particle.
- Mass and atomic numbers have changed. From the equation, we can see that both the mass number and the atomic numbers have changed. The mass number has decreased by 4, whilst the atomic number has decreased by 2.
Beta Decay
Now, let us look at beta decay with this example:
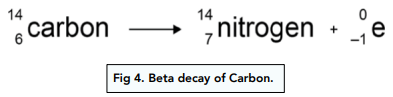
- An electron has been emitted. Since an electron has been emitted, we know that this is beta decay. The electron is the beta particle.
- Only the atomic number has changed. From the equation, we can see that only the atomic number has changed. The mass number does not change, since the electron has a negligible mass.
Gamma Rays
As previously mentioned, gamma rays are a form of electromagnetic radiation. Unlike beta and alpha decay, gamma rays do not cause a change in mass or charge.
This means that neither the atomic number or the mass number changes when gamma rays area emitted.
Study Mind Tip: Although we do not need to write out equations for gamma decay, AQA exams will expect you to identify when radiation is gamma decay rather than alpha or beta. Similarly, you will be expected to know why gamma decay is often more dangerous than alpha or beta.
AQA Specification: Students should be able to use the names and symbols of common nuclei and particles to write balanced equations that show single alpha (α) and beta (β) decay. This is limited to balancing the atomic numbers and mass numbers. The identification of daughter elements from such decays is not required.
Writing Equations to Show Decay
Question: An isotope of barium is Ba-139. Ba-139 decays by beta decay to lanthanum-139 (La-139).
Complete the nuclear equation that represents the decay of Ba-139 to La-139.
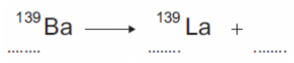
- We know that the Barium is undergoing beta decay, not alpha decay.
- Now, we need to find the atomic number for barium. We can do this by looking at a periodic table.

- From this, we can see that Barium has an atomic number of 56. Therefore, our first gap must be 56.
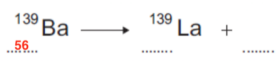
- Next, we need to fill in the particle that is emitted in beta radiation. This is the beta particle:
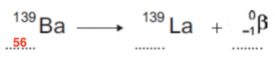
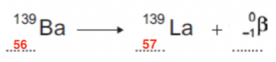
- Once we have filled this in, we just need to balance both sides of the equation.





Still got a question? Leave a comment
Leave a comment